Intraventricular cavernoma
Images



Intraventricular cavernoma
Findings
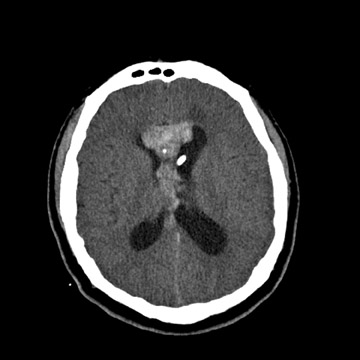
A non-contrast computed tomography (CT) head exam demonstrated a hyperdense midline intraventricular lesion located along the septum pellucidum, extending into the frontal horns of both lateral ventricles and to the level of the foramen of Monro (Figure 1). Aventricular drain, which had been placed at our institution, following review of an outside CT exam (not shown), terminated in the left lateral ventricle. There was mild hydrocephalus with the left lateral ventricle asymmetrically larger in size as compared with the right, possibly due to entrapment.
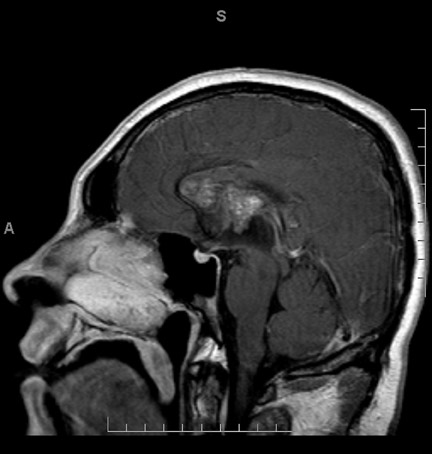
Magnetic resonance imaging (MRI) was performed for better characterization of the lesion (Figure 2). On T1-weighted (T1W) images, the mass demonstrated heterogeneous signal with areas of isointense signal as compared with that of the brain parenchyma and scattered areas of more focal hyperintense signal within the lesion. T2-weighted (T2W) images demonstrated a peripheral rim of signal loss surrounding the lesion with heterogeneous isointense and hyperintense signal within the lesion. Periventricular T2 hyperintense signal, predominantly along the frontal horn of the left lateral ventricle, was compatible with transependymal resorption of cerebrospinal fluid. On gradient recalled echo (GRE) imaging, the mass demonstrated blooming artifact compatible with hemosiderin deposition. There was no enhancement of the lesion following IV contrast administration. There were no intraparenchymal lesions,including no areas of blooming artifact,on GRE to indicate additional small focal sites of hemosiderin deposition. Cerebral angiography was normal (not shown) with no angiographically visible vascular malformation, aneurysm, mass or mass effect.
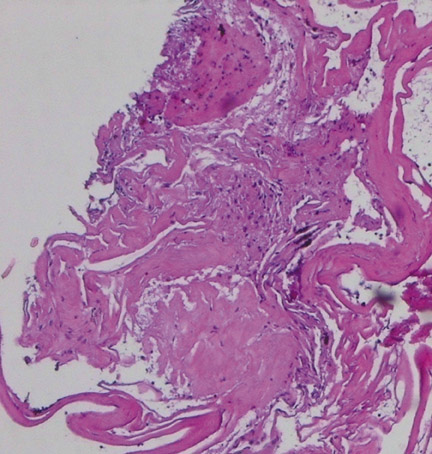
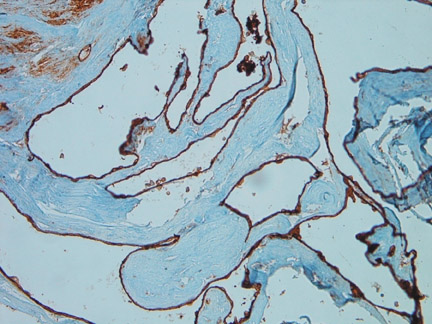
Histological evaluation from endoscopic biopsy of the lesion demonstrated multiple spaces of varying size surrounded by a fibrous wall and lined by a single layer of endothelial cells (Figure 3). The lining cells were strongly immunoreactive for CD31, supporting their endothelial nature (Figure 4). The adjacent brain tissue demonstrated astrocytosis and evidence of old hemorrhage, characterizedby blood degradation products including hemosiderin.
Discussion
Intraventricular cavernomas (IVC) are rare entities that have not been described in detail in the radiological literature. Several reports and descriptions have been periodically published in the neurosurgical literature with a surgical emphasis on successful excision.
Intraventricular mass lesions represent approximately 10% of all CNS neoplasms.1 These lesions are comprised of a diverse group of entities. An appropriate differential diagnosis can usually be formulated based on the radiological appearance, location, and the age of the patient. Symptoms are usually non-specific and are related to mass effect with obstruction and resultant hydrocephalus. Though surgery is the primary choice of treatment for most of these lesions including IVC, accurate preoperative diagnosis can significantly aid the surgical procedure and therefore decrease morbidity, residual disease, and recurrence, resulting in a better overall outcome. There is no demonstrated benefit for radiotherapy or radiosurgery in the treatment of IVC.
Cavernous angiomas are the most commonly identified vascular malformation, which is a class of benign entities that also includes arteriovenous malformations, developmental venous anomalies, and capillary telangiectasias. They are found in all age groups without sex predilection. A series study by Curling et al. found a 0.4% incidence in a group of unselected patients.2 They are most often found in the subcortical brain parenchyma, the deep cerebral white matter and the basal ganglia.3 Pathologically, cavernomas are multilobulated berry-like lesions that contain hemorrhage in various stages of evolution and are composed of closely approximated endothelial-lined sinusoid spaces without significant intervening neural tissue.1 Clinically, patients can present with headache, seizure or focal neurologic deficit. Repeated occult hemorrhage is common.
IVCs are rare entities. The prevalence rate of IVC varies between 2.5% to 10.8%.4 A summary of 45 reported cases in the literature by Reyns et al. found that 44% of intraventricular cavernomas were located in the third ventricle, 27% in the lateral ventricle, 20% in the trigone, and 9% in the fourth ventricle.5 Clinical presentation is usually non-specific and may include seizure, memory loss,headache, paresthesia, and hemiparesis.5-9 Symptoms have been postulated to be secondary to increased intracranial pressure withresultant mass effect on vital surrounding structures.5,9 Intermittent or fluctuating symptoms have also been described secondary to minor hemorrhages within the ventricles.7 Hydrocephalus is not present in many cases and primarily depends on location.7
IVCs have been found to be more voluminous than their intraparenchymal counterparts and can measure up to several centimeters. It has been suggested that the surrounding cerebrospinal fluid and the lack of restriction from adjacent brain parenchyma allows for more unrestrictive growth.8 Pseudotumoral growth of the mass can arise from repetitive intralesional hemorrhage.3
On imaging,IVCs may show a similar appearance to that of intraparenchymal cavernoma. On CT they are intrinsically hyperdense on unenhanced exams with specks of calcification.5,6 Following intravenous contrast, enhancement can vary from none to avid.5,7,9 MR imaging demonstrates a well-defined mass with heterogeneous signal intensity on both T1W and T2W sequences.5-9 The hypointense areas correlate with areas of calcification and fibrosis while the hyperintense areas correlate with areas of methemoglobin.5 These lesions also demonstrate a hypointense peripheral rim on T2W images due to paramagnetic effect and gliotic reaction towards hemosiderin.5-7 GRE imaging is very sensitive in demonstrating the susceptibility effect of hemosiderin and is indispensable in identifying the multiplicity of the lesion and therefore supporting the diagnosis of IVC.
On imaging, various intraventricular lesions may be mistaken for IVC, including intraventricular arteriovenous malformations, low-grade astrocytomas, meningiomas, teratomas, or neurocytomas. Intraventricular location, size, hyperintensity and partial calcificationmay suggest a neoplastic lesion. Lack of surrounding edema on FLAIR or T2W imaging, peripheral hemosiderin, and lack of enhancement make tumor less likely and favor atypical vascular malformation. But on MRI, central hyperintensity,due to methemoglobin and peripheral hemosiderin rim, limits the differentials. Contrast enhancement does not help much in differentiation. GRE may be extremely helpful. Cavernomas are angiographically occult malformations because their connection with the vascular system is generally poor. On angiography they appear as a non-specific avascular mass or rarely may show a blush, enlarged, prominent posterior choroidal artery with draining vein.
Conclusion
Intraventricular cavernomas are uncommon. An incorrect preoperative diagnosis can result in inefficient treatment, such as radiotherapy, for this surgically curable benign lesion. Compared with intraparenchymal cavernoma, intraventricular cavernoma often demonstrates less characteristic radiological appearance. However, correct analyses with susceptibility weighted imaging, such as GRE, facilitate the differentiation and support the correct diagnosis, encouraging appropriate surgical treatment. Therefore, it is important to be able to characterize these lesions on radiological imaging. Differentiating this lesion from a tumor, or from other entities, is possible based on MRI and is important in guiding the necessary treatment.
- Osborn AG, Rauschning W. Brain tumors and tumorlike masses: Classifications and differential diagnosis. In: Osborn, AG. Diagnostic Neuroradiology. St Louis,Mo:Mosby;1994:422-436.
- Curling OP, Kelly DL, Elster AD, et al. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702-708.
- Kaim A, Kirsch E, Tolnay M, et al. Foramen of Monro mass: MRI appearances permit diagnosis of cavernous haemangioma. Neuroradiology. 1997;39:265-269.
- Simard JM, Garcia-Bengochea F, Ballinger WE, et al. Cavernous angioma: A review of 126 collected and 12 new clinical cases. Neurosurgery. 1986;18:162-172.
- Reyns N, Assaker R, Louis E, et al. Intraventricular cavernomas: Three cases and review of the literature. Neurosurgery. 1999;44:648-654.
- Anderson RC, Connolly ES Jr, Ozduman K, et al. Clinicopathological review: Giant intraventricular cavernous malformation. Neurosurgery. 2003;53:374-378.
- Itoh J, Usui K. Cavernous angioma in the fourth ventricular floor. Neurol Med Chir (Tokyo). 1991;31:100-103.
- Nieto J, Hinojosa J, Munoz MJ, et al. Intraventricular cavernoma in pediatric age. Childs Nerv Syst. 2003;19:60-62.
- Kumar GS, Poonnoose SI, Chacko AG, et al. Trigonal cavernous angiomas: Report of three cases and review of literature. Surg Neurol. 2006;65:367-371.
Related Articles
Citation
Intraventricular cavernoma. Appl Radiol.
November 17, 2010