FDA clears Siemens MR breath-holding software
June 6, 2013 - Siemens Healthcare announced that the U.S. Food and Drug Administration (FDA) recently approved new magnetic resonance (MR) software that reduces patient MR breath-hold up to 50% without sacrificing image resolution, enabling ultra-fast, high-quality 3-dimensional (3D) T1 imaging.
The company’s CAIPIRINHA (Controlled Aliasing in Volumetric Parallel Imaging Results IN Higher Acceleration) software is part of Siemens’ syngo MR D13A software package for parallel MR imaging.
“Before we got CAIPIRINHA our breath hold typically ran from 18 to 24 seconds on the sequences that CAIPIRINHA applies to, and we’ve been able to reduce some of the sequences down to about 14 seconds or less for 3D T1 sequences,” said Mustafa R. Bashir, MD, Director of MRI, Assistant Professor of Radiology, Division of Abdominal Imaging, Department of Radiology, Duke University Medical Center.
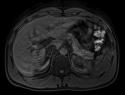
A.) 25-second breath hold without CAIPIRINHA, with motion from patient unable to hold breath.
B.) 7-second breath hold with CAIPIRINHA (no motion).
“For arterial phases in the abdomen, we do three fast 3D T1w acquisitions in a single breath hold in order to provide some redundancy in the passage of the contrast bolus. That triple phase sequence was traditionally as long as 25 to 28 seconds and was relatively low-resolution, and we have been able to reduce it to 23 seconds for 3 images with higher resolution. The image quality and sharpness has clearly improved,” said Dr. Bashir. “So for most VIBE sequences, we have taken the speed advantage and used that to provide an easier breath hold for the patient.”
Siemens’ CAIPIRINHA software enables acquisition of higher-quality 3D volumetric interpolated breath-hold sequence (VIBE) T1 images through higher acceleration factors. CAIPIRINHA software is included as standard with Siemens’ D13A software for MAGNETOM Aera 1.5 Tesla, MAGNETOM Skyra 3T, MAGNETOM Avanto 1.5T, and MAGNETOM Verio 3T systems. It is also available with Siemens’ D14 software for the MAGNETOM Essenza 1.5T system.
For more information: usa.healthcare.siemens.com and www.appliedradiology.com
Related Articles
Citation
FDA clears Siemens MR breath-holding software. Appl Radiol.
June 6, 2013