Pulmonary arteriovenous malformation (AVM)
Images
Pulmonary arteriovenous malformation (AVM)
Findings
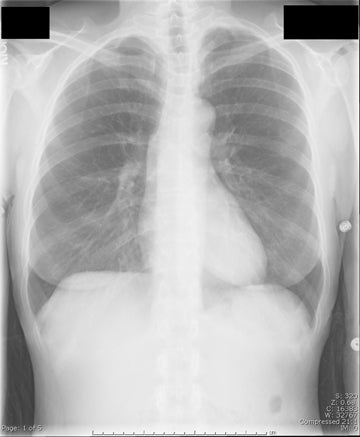
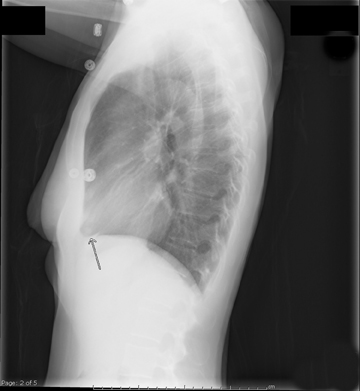
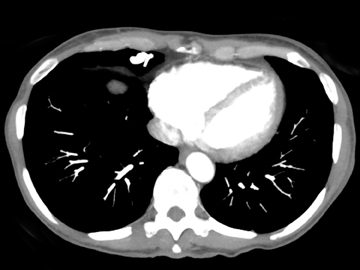
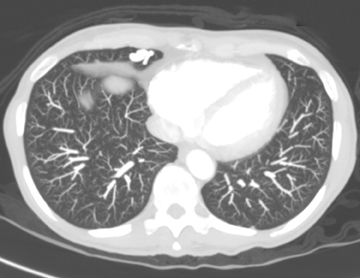
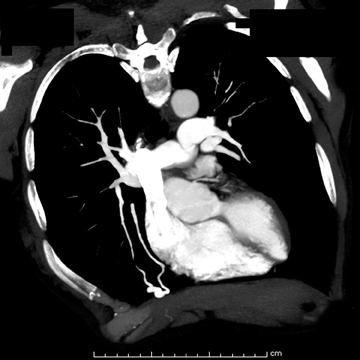
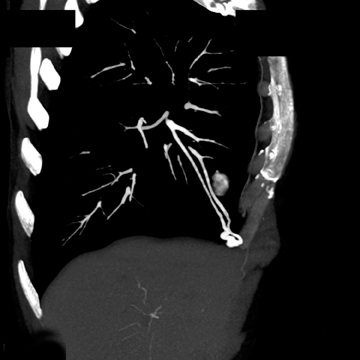
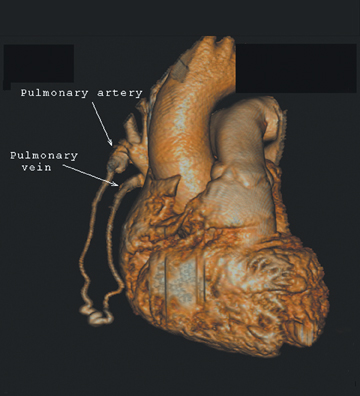
The patient initially underwent PA/lateral chest radiography (Figure 1). A nodular opacity was detected anteriorly on the lateral view. It was not well visualized on the frontal projection. For further evaluation, a contrast-enhanced chest computed tomography (CT) was performed, confirming the presence of a lobular, high-density lesion in the right middle lobe measuring 8 mm in diameter (Figure 2). Multiplanar reformatted images confirmed the suspected diagnosis of pulmonary arteriovenous malformation (AVM) by establishing vascular communication with the lesion, with the origin and termination of the pulmonary AVM at the branches of the right pulmonary artery and vein, respectively (Figure 3). Volume rendering was performed to depict the anatomy more clearly (Figure 4).
Discussion
AVMs represent abnormal communications between the pulmonary arterial and venous systems that bypass the capillary bed. Their frequency varies, occurring in roughly 10 to 20 persons per 100,000.1 Pulmonary AVMs may be classified as simple with a single feeding and draining vessel (80% of cases), or complex, with 2 or more feeding or draining vessels (20% of cases). Up to 65% of pulmonary AVMs are found in the lower lobes of the lung.2
It has been reported that anywhere from 50% to 90% of pulmonary AVMs are associated with hereditary hemorrhagic telangiectasia(HHT), an unusual autosomal dominant disorder also referred to as Rendu-Osler-Weber syndrome. This condition affects the arteries of the nose, skin, brain, lung, and gastrointestinal tract.1 Multiple pulmonary AVMs convey a higher association with HHT.2 The percentage of HHT patients with pulmonary AVMs ranges from 5% to 33%.3 Pulmonary AVM screening is recommended for families with HHT, and screening includes chest radiography and arterial blood gas measurements taken in the upright and supine positions.3
Pulmonary AVMs frequently go unrecognized until the late teens, but they may remain asymptomatic throughout life.3 Pulmonary symptoms include dyspnea, fatigue, cyanosis, and orthodeoxia (decreased arterial oxygen content while upright), all due to right-to-left shunting of blood through the pulmonary AVM.4 The most serious complications of pulmonary AVMs are potentially fatal hemoptysis or hemothorax (in up to 10% of patients).1,2 This risk is reportedly greater in pregnant women during childbirth.5 Neurologic sequelae may develop asa result of the unfiltered passage of paradoxical emboli through the pulmonary system to the brain. Brain abscess may also result following dental procedures, for instance.4,6 Neurologic symptoms may be the presenting symptoms in up to 40% of patients.5
On chest radiography, pulmonary AVMs may be seen as rounded, circumscribed pulmonary nodules. Feeding vessels may or may not be visualized. On chest CT, a homogeneous, circumscribed, noncalcified nodule may be seen. Likewise, a serpiginous mass may be seen with connections to vascular structures.7 Three-dimensional reconstructions may be used to delineate the vascular nature of the lesion, identifying the points of origin of feeding and draining vessels. The differential diagnosis for such a finding on chest radiograph or axial-CT images include other solitary pulmonary nodules, such as bronchogenic carcinoma and metastatic disease,hamartoma, tuberculoma, and bronchogenic cyst, to name a few.3 Thorough evaluation of such findings with 3-dimensional multiplanar imaging is valuable, as many solitary pulmonary nodules must undergo biopsy to exclude malignancy, a procedure that could be catastrophic for a patient with a pulmonary AVM.
Studies have shown that CT is the best noninvasive modality for evaluation of pulmonary AVMs. However, pulmonary angiography is necessary before embolotherapy or other intervention is undertaken.4,7
Pulmonary AVM treatment is recommended for symptomatic patients or those AVMs with a feeding artery diameter ≥3 mm.1,2,5 Transcatheter embolotherapy with stainless steel coils or detachable balloons is most commonly performed.5 Following embolotherapy, a considerable decrease in pulmonary AVM size is expected; persistent size may indicate persistent perfusion. Furthermore, it has been suggested that smaller malformations or smaller feeding vessels may become enlarged after successful embolotherapy of larger AVMs.7 Therefore, long-term follow up, including chest CT examinations every 1 to2 years, is recommended.5
Conclusion
Pulmonary AVM is a relatively rare disease, which is frequently associated with HHT. Differentiation of this condition from other pulmonary nodules is essential to avoid potentially catastrophic biopsy. Three-dimensional multiplanar imaging, clearly defining the vascular nature of the lesion, is a valuable tool in the accurate diagnosis of pulmonary AVM.
- White RI, Pollak JS. Pulmonary arteriovenous malformations: Diagnosis with three-dimensional helical CT—a breakthrough without contrast media. Radiology. 1994;191:613-614.
- White RI, Lynch-Nyhan A, Terry P, et al. Pulmonary arteriovenous malformations: Techniques and long-term outcome of embolotherapy. Radiology. 1988;169:663-669.
- Kjeldsen AD, Oxhoj H, Andersen PE, et al. Pulmonary arteriovenous malformations: Screening procedures and pulmonary angiography in patients with hereditary hemorrhagic telangiectasia. Chest. 1999;116:432-439.
- White RI. Pulmonary arteriovenous malformations: How do we diagnose them and why is it important to do so? Radiology. 1992;182:633-635.
- Dinkel HP, Triller J. Pulmonary arteriovenous malformations: Embolotherapy with superselective coaxial catheter placement and filling of venous sac with Guglielmi detachable coils. Radiology. 2002;223:709-714.
- Guttmacher AE, Marchuk DA, White RI. Sum & substance. Hereditary hemorrhagic telangiectasia. Radiology. 1997;204:342.
- Remy J, Remy-Jardin M, Wattinne L, et al. Pulmonary arteriovenous malformations: Evaluation with CT of the chest before and after treatment. Radiology. 1992;182:809-816.
Related Articles
Citation
Pulmonary arteriovenous malformation (AVM). Appl Radiol.
April 28, 2011