MR imaging of abdominal and pelvic pain in pregnancy
Images
Dr. Bailey is a Radiology Resident, Dr. Twickler is a Professor of Radiology and Obstetrics/Gynecology, Vice Chair for Academic Affairs, and Holder of the Fred Bonte Professorship in Radiology, and Dr. Rofsky is a Professor and Chairman of the Department of Radiology, at the University of Texas Southwestern Medical Center at Dallas, Dallas, TX. Dr. Pedrosa is Associate Professor of Radiology, Harvard Medical School, and Director of Body MRI, Department of Radiology, Beth Israel Deaconess Medical Center, Boston, MA. Dr. Rofsky also serves as a Consultant to Bioclinica.
Imaging frequently guides patient management in the evaluation of pelvic pain. When the patient is also pregnant, the source of the patient’s pain is often clinically confounded. An accurate imaging diagnosis is important, since surgical and medical management decisions often rely on those findings. However, the anatomic and physiologic changes associated with pregnancy pose substantial challenges to the interpreting radiologist. Ultrasound should be used as initial evaluation given its lack of ionizing radiation and wide availability. When ultrasound evaluation is equivocal or limited, cross-sectional imaging is often requested typically with computed tomography (CT).
With a heightened awareness of radiation exposure and faster, motion immune sequences, magnetic resonance (MR) imaging is being used with increased frequency.1-5 MR of the abdomen with a gravid uterus displacing familiar anatomy can be challenging for the otherwise seasoned radiologist well versed in cross-sectional imaging.1 In this review, we will present practical applications of MR in the pregnant patient with pelvic pain, knowing that rapid diagnosis and prompt treatment is of the utmost importance in this population.6
Safety
MR considerations
While the lack of ionizing radiation characteristic of MR imaging represents an obvious safety advantage for imaging pregnant patients, it is important to consider exposure to the electromagnetic fields that are encountered. Numerous tissue, cell culture, and animal experiments have failed to reveal adverse impacts resulting from such exposure.7-14 Concerns for heat deposition are most relevant at the skin, and even then, whenMR imaging is performed within the FDA guidelines for specific absorption rate (SAR) the risk is minimal and negligible at the body core.15 A study using volunteers with fluid-filled stomachs suggests sound intensity levels are attenuated to a safe level by the amniotic fluid.9 Fetal heart rate and fetal movement patterns during MR procedures are unchanged with comparison to baseline.10 Studies following children imaged in utero have also demonstrated no increased rate of disease or disability.11,12
The American College of Radiology considers MRI safe during pregnancy and acceptable to use when the imaging results will affect clinical management of the fetus or mother during the pregnancy.8,13,16 During the first trimester, more judicious use of MR is recommended because of organogenesis; early research suggested disorders of embryogenesis and teratogenic effects in animal studies.11,16,17 However, to date there are no known negative effects in the human population. Current recommendations are to explain the risks and benefits of the exam to the mother prior to imaging and to obtain written consent.
MR contrast agents
The widely used extracellularly distributed, gadolinium-based intravenous contrast agents cross the placenta in primate studies and gadolinium-chelate molecules remain for an indeterminate time within the amniotic fluid.16,18 During that time, there is potential for dissociation and release of toxic, free gadolinium ions, which the fetus is repeatedly exposed to as it swallows and excretes.8 Intravenous MR contrast should not be routinely used in pregnancy, as gadolinium-based contrast agents are considered category C drugs by the FDA.
Oral contrast preparations containing barium sulfate are not systemically absorbed in animal models.19 Gastrointestinal absorption of iron from oral MR contrast agents containing iron oxide (such as Ferumoxil) is variable, but animal studies have not demonstrated detrimental effects at doses multiplicatively exceeding the expected concentrations achieved with specified dosing for human use. According to package information of oral contrast agents, studies have not been done on pregnant humans and iron oxide MR contrast agents are considered category B drugs by the FDA.
MR techniques
Standard MR sequences that we use for pregnant patients with abdominal pain include motion insensitive T2-weighted images, single shot fast spin echo (SSFSE or HASTE) in three planes as well as fat-saturated SSFSE images in the axial plane. Also, axial 2D TOF (to identify thromboses and for anatomical differentiation) and axial T1-weighted GRE (in- and out-of-phase to assist in characterization of hemorrhage) are included.2 The use of diffusion-weighted imaging is gaining popularity in the diagnosis of inflammatory conditions, such as appendicitis, in the abdomen.20
Given the often urgent condition of many patients, there has been interest in and success using MR evaluation without oral contrast agents.21,22 Several studies, however, consider negative oral contrast agents helpful in anatomic differentiation during pregnancy3 and diagnostically advantageous when used as a problem-solving tool.23-26 For the majority of our studies, an oral preparation using 300 mL Gastromark (Mallinckrodt Medical, St Louis, MO) and 300 mL Readi-cat 2 (Bracco Diagnostics, Princeton, NJ) given 1.5 hours prior to examination was used to provide negative contrast within the bowel lumen without substantial susceptibility effect.2 While outcomes assessments have been reported for series with oral contrast,1 at the time of this writing, studies without oral contrast with similar rigor have not been reported.
Gastrointestinal assessment
Appendicitis
Appendicitis is the most common cause of abdominal pain in pregnant patients requiring surgical intervention, and it occurs at a frequency comparable to that of the general population (0.02% - 0.07%).1 The risk of perforated appendicitis is greater in pregnant patients, possibly due to delay in diagnosis. Efficient, accurate diagnosis is paramount as complications of appendicitis include increasing rates of maternofetal morbidity and mortality. Because surgical intervention alone imparts increased fetal mortality (3%), every effort should be made to prevent unnecessary intervention.27,28
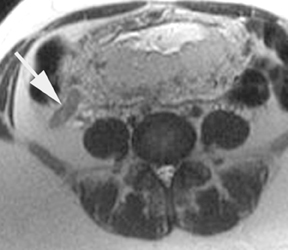
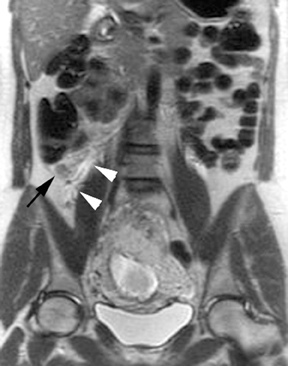
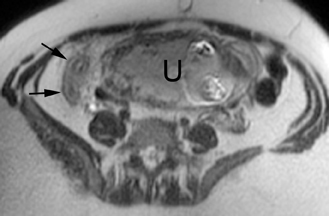
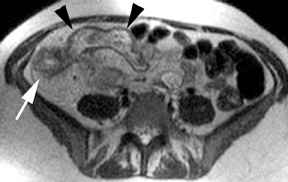
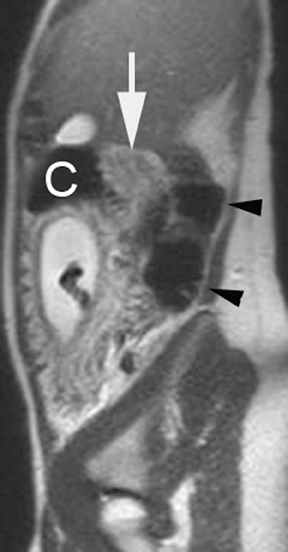
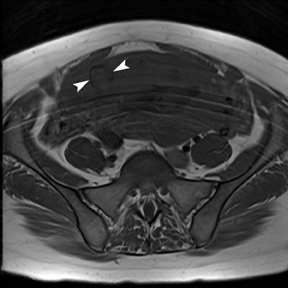
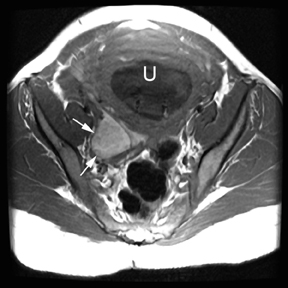
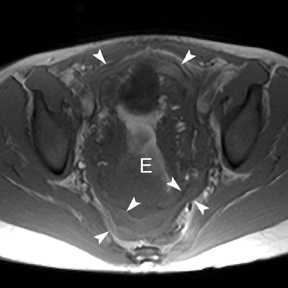
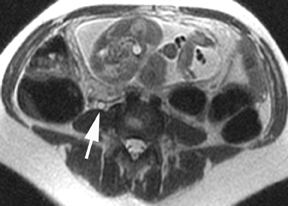
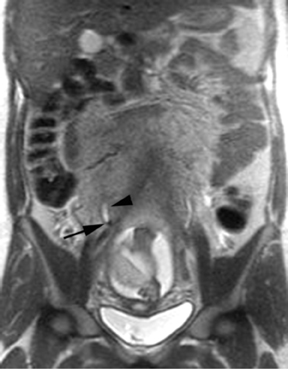
The criteria for diagnosing appendicitis with MR are similar to that for using CT, including a fluid-filled appendix of >6 mm to 7 mm indiameter and ancillary findings of inflammation.1,13 However, identifying the appendix can be challenging on MR, as well as in the gravid abdomen. It is important to recognize that, as the uterus enlarges with pregnancy, the appendix tends to migrate cephalad and medially.2 When identifying the appendix, verify its location using all 3 imaging planes and a localizing or “cross referencing” tool where available(Figure 1).29
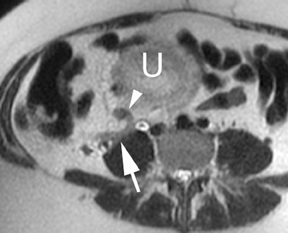
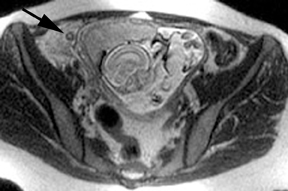
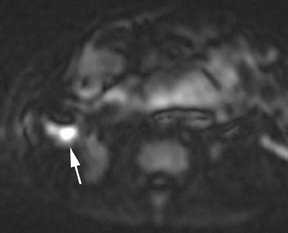
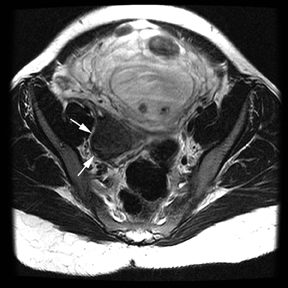
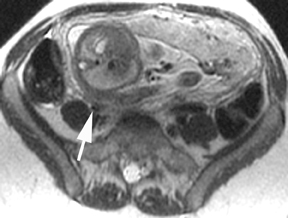
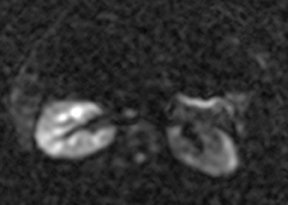
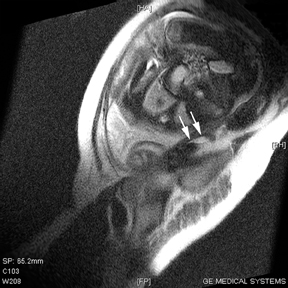
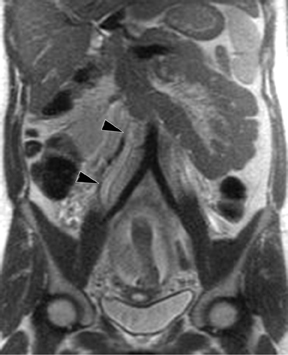
Oral contrast or air will fill the normal appendix and appear dark on T2-weighted images. Fat-saturation pulses are invaluable when differentiating peri-appendiceal inflammation (high signal fluid) from fat (high signal on T2-weighted images without fat saturation). Furthermore, the demonstration of peri-appendiceal fluid can be helpful in suggesting appendicitis in otherwise equivocal cases, whereas th eabsence of peri-appendiceal fluid can be helpful in ruling out appendicitis when the appendix cannot be visualized.1,3 Detection of inflammation is particularly important when the appendix is not well seen, borderline dilated (early appendicitis) or in cases of perforation.2 Restricted diffusion is seen as high signal intensity in inflamed appendices on diffusion-weighted imaging (Figure 2).20 Since dilated gonadal vessels can mimic the appearance of the appendix on T1- and T2-weighted images, time-of-flight (TOF) images are recommended to distinguish between the high signal of vessels and low signal intensity of the appendix (Figure 3).2
Inflammatory bowel
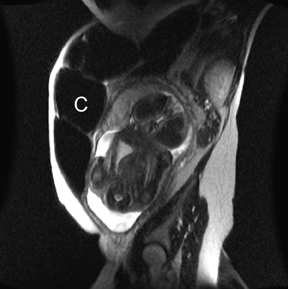
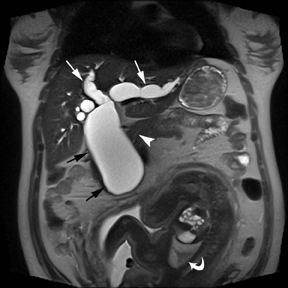
MR can be used to evaluate the intestinal tract and is sensitive for detecting the early inflammation seen in active flares of inflammatory bowel disease (IBD). Bowel-wall edema is readily identified on MR using single shot, T2, fat-suppressed images and balanced steady-state, free-precession (bSSFP) images. However, this finding is not specific to IBD; it can also be seen in various enteritidies/colitidies, enteropathies, and ischemic insults (Figure 4). As in other modalities, MR often cannot completely differentiate infectious from inflammatory conditions of the bowel;however, the distribution and affected segments (eg, the terminal ileum is a typical target of inflammatory bowel, skip lesions versus continuous involvement from the rectum) and signs, such as mesenteric inflammation related to dilated vasa rectae, may aid in diagnosis. The sequelae ofIBD, such as inner-loop abscesses (very high T2 signal, more easily seen if negative oral contrast agents are used to differentiate from fluid filled bowel26), fluid- or gas-filled fistulae, and luminal strictures, can also be identified with MR.
Bowel obstruction
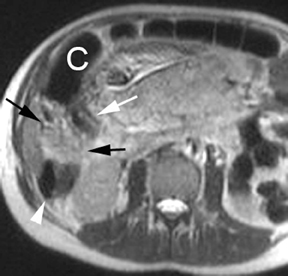
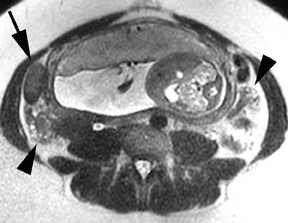
Although the etiology of bowel obstruction may not be readily apparent on MR imaging, the diagnosis can be reliably made. Small bowel is considered dilated at 3-cm transverse diameter and colon is considered dilated beyond 6 cm (cecum >9 cm) as with other modalities (Figure 5). A keen search is advised when assessing for the signal void of gas, as can be seen in the setting of pneumatosis or free air; extraluminal gas is more difficult to identify on MR compared to CT. Adhesions and hernias are the most frequent causes of bowel obstruction.2 Excellent soft-tissue differentiation is possible using single-shot, echo-train, spin-echo, T2-weighted images when oral contrast is given and motion artifact is minimized by otherwise fasting the patient prior to examination (to decrease peristalsis).
Diverticulitis
Bowel-wall thickening and diverticular abscesses can be identified using similar sequences and methods as described above and do not strictly require intravenous contrast. On non-fat suppressed, T1-weighted images, inflammatory fluid will be of low signal intensity and standout in comparison to pericolonic fat (Figure 6). Diverticula are more common in the sigmoid and descending colon. It is also worth emphasizing that focal thickening from diverticulitis may, at times, be difficult to differentiate from colonic neoplasm; in those instances a follow-up assessment for resolution is advised. However, for women of childbearing age colonic neoplasms are rare.
Biliary assessment
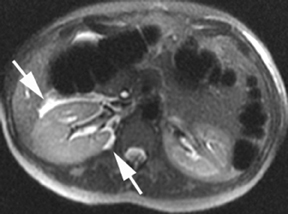
MR is excellent for visualization of the biliary tree by using MR cholangiopancreatography (MRCP) to accurately diagnose a wide range of abnormalities and to facilitate surgical planning. The SSFSE sequence used in evaluating the pregnant patient is a common staple of MRCP examinations and an assessment of the biliary tract is inherent to the protocol, almost always captured with the large, coronal field-of-view. MRCP is of particular value when the anatomy is unfavorable for adequate sonographic visualization (as is frequently encountered in pregnancy) (Figure 7). Biliary stones and biliary gas both produce signal void on MR; however, nondependent positioning suggests biliary gas. Inflammation of the biliary system appears as wall thickening and edema, and can be associated with luminal dilation, adjacent fat stranding and fluid collections. The full extent of biliary neoplasm, stricture or malformation can be readily identified using MR. Oral contrast helps to delineate anatomy near the pancreatic head,30,31 but if prior sphincterotomy of the major or minor papillae has been performed, the negative contrast can reflux into the biliary system and obscure visualization of the biliary tree.32
Gynecologic assessment
Degenerative fibroids
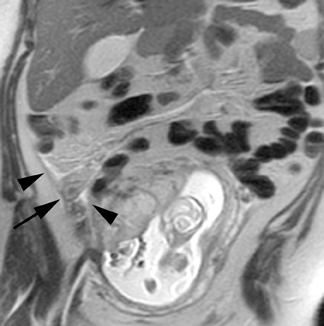
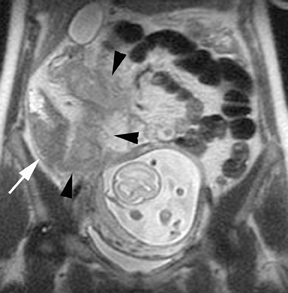
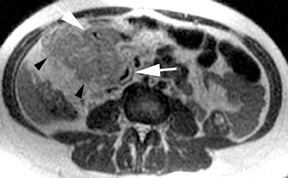
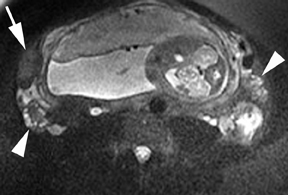
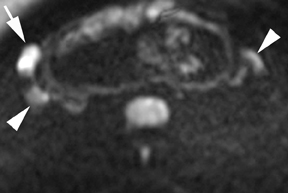
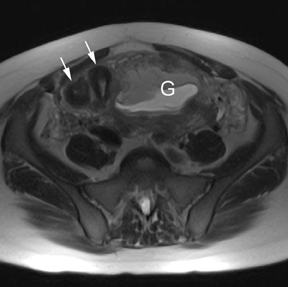
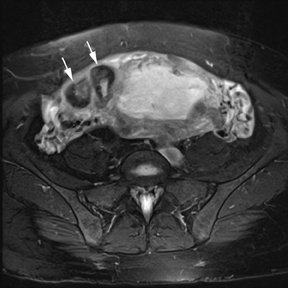
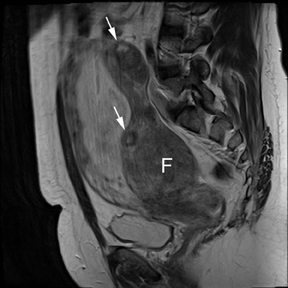
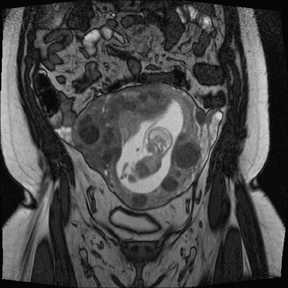
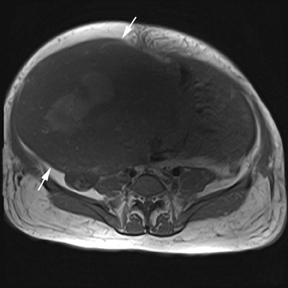
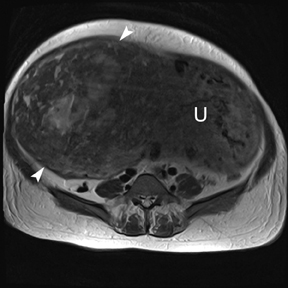
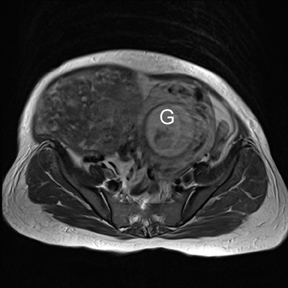
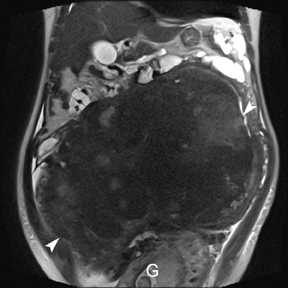
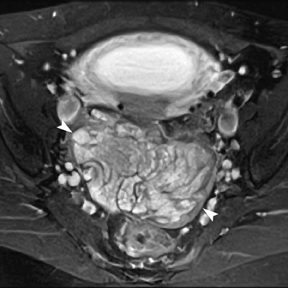
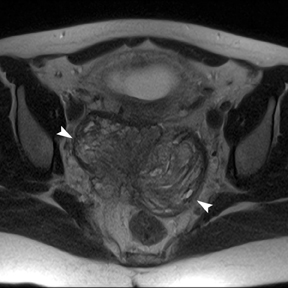
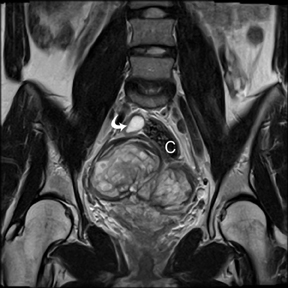
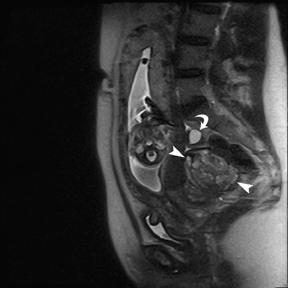
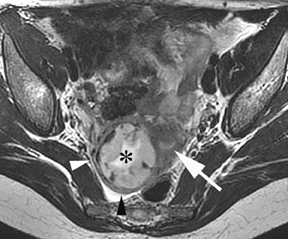
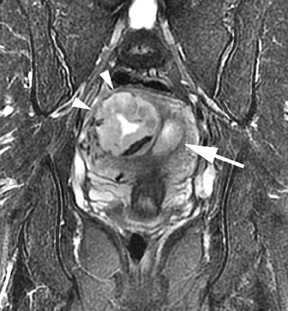
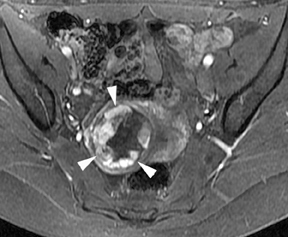
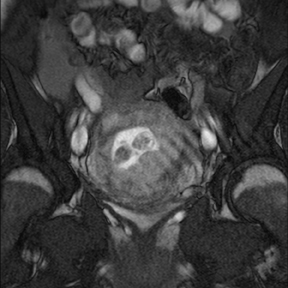
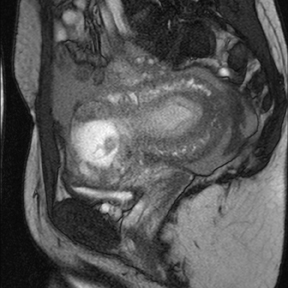
Leiomyomas (fibroids) are benign, smooth muscle neoplasms extending from or involving the myometrium of the uterus. Fibroids that rapidly grow during pregnancy may cause pain. Pain can also be associated with torsion of pedunculated fibroids and degeneration if the fibroids outgrow their blood supply. Hemorrhagic infarction or “red” degeneration is specifically associated with pregnancy and demonstrates high T1signal due to hemorrhagic components that may be diffuse or peripheral. Signal on T2-weighted imaging is often variable; however, high T2signal located centrally in the fibroid is consistent with necrosis.2,33 Diffusion-weighted imaging can be performed to evaluate for the restricted diffusion of acute degeneration in the fibroid (Figures 8-10).
Ovarian masses
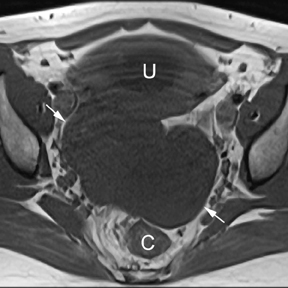
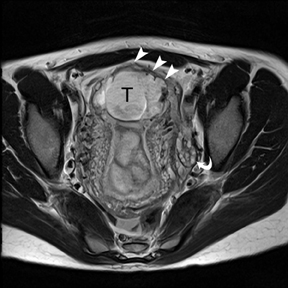
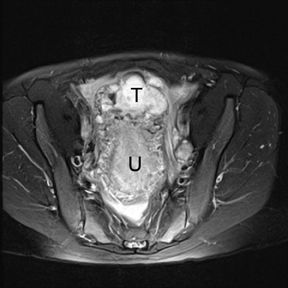
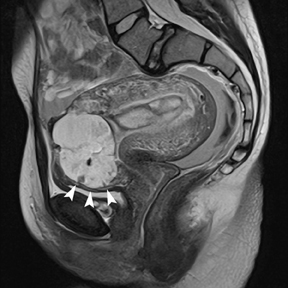
If a pelvic mass is identified during sonographic examination, compression can determine if the mass is associated with or separate from the ovary. However, in pregnancy, the actual location and characterization of ovarian masses may be problematic. MR is particularly helpful in defining the relationship of masses to the uterus and ovary and can offer tissue characteristics based on the T1- and T2-weighted signal (Figure 11).
Acutely, ovarian torsion may cause enlargement and edema of the affected ovary, which may be more easily detected on fat-saturated,T2-weighted images. An ovarian mass or cyst is often associated with cases of ovarian torsion. Hemorrhage within the ovarian stroma itself is a later finding of torsion and demonstrates variable signal on T1- and T2-weighted images, depending on the age of the contained blood products.2
Endometriosis
Although endometriosis is a common cause of infertility, pain related to endometriomas can be encountered during pregnancy. Endometriomas will demonstrate high signal on T1-weighted images and typical “shading” (low signal on T2-weighted images), as seen in nonpregnant patients. Multifocal involvement, adhesion, and tethering of adjacent structures also support a diagnosis of endometriosis (Figure 12).2
Atypical ectopic pregnancy
Ninety-five percent of ectopic pregnancies are tubal and can be diagnosed with ultrasound. In the setting of atypical locations and large ectopics, the large field of view afforded by MR is helpful in defining the anatomic origin and effect on other structures in the pelvis (Figure13). One example is the case of a cesarean ectopic in which the relation to the cesarean scar can clearly be seen (Figure 14).34
Urologic assessment
Cystitis and pyelonephritis
Again, with urologic conditions, ultrasound is the primary imaging modality for diagnosis in pregnant patients. However, thickening of the urinary bladder wall can be detected on MR as well as on CT and may suggest inflammation related to infection. Nondependent signal voids in the urinary bladder suggest air, which is only normal if iatrogenic (eg, recent Foley catheter placement or cystoscopy). Pyelonephritis is usually a complication of lower urinary tract infections. The affected kidney may be enlarged or have perinephric fluid, which is nonspecific. The prototypical “striated nephrogram” will not be detected without intravenous contrast, but diffusion-weighted imaging may identify restricted diffusion (Figure 15). Proteinaceous material in the collecting system will be of higher signal intensity than urine on unenhanced T1-weighted images. Renal abscesses may complicate pyelonephritis; they are difficult to confidently identify on MR without intravenous contrast. A focal area of perinephric stranding (versus perinephric stranding surrounding the whole kidney) is a clue to a possible underlying abscess.33
Urinary obstruction
Using half-Fourier single-shot fast SE for evaluation of the urinary tract, collecting system, and ureteral stones will be detected as focal areas of signal void. Similarly, blood clots and fungus balls are also of low signal intensity. Collecting system air should layer nondependently. When there is laminar flow in the ureter, a central, poorly defined filling defect may be seen on the SSFSE sequence (Figure 2). Perirenal fluid can be seen in obstruction, but is nonspecific, as in other radiographic modalities.2 Urothelial tumors are less common causes of urinary obstruction and may be eccentrically located within the collecting system and have associated wall thickening. The full extent of tumor involvement may be difficult to delineate without intravenous contrast.33
Hydronephrosis is a common finding late in pregnancy. If the degree of hydronephrosis is moderate to severe, or the patient is symptomatic, MR can further evaluate the ureters. Typically, pregnancy-related hydronephrosis will show tapering of the ureter to the point where it is extrinsically compressed by the gravid uterus and posterior structures, often at the sacral promontory (Figure 16). Ureteral calculi are typically symptomatic, will cause a more abrupt caliber change of the ureter, and may have associated periureteral inflammatory changes (Figure 17). If ureteral dilation is distal to the pelvic inlet, there is an increased likelihood of an obstructing calculus being the cause of hydronephrosis.2
Conclusion
Determining the source of abdominal and pelvic pain in the pregnant patient necessitates an accurate and timely diagnosis. Imaging will continue to provide vital diagnostic information; the utilization of MR technology is growing in cases where sonographic evaluation alone is not sufficient to guide management.13 An awareness of the utility of MR imaging for the pregnant patient with abdominal and pelvic pain and an appreciation for the appearances of a variety of pathologies can be useful for enabling a broader implantation of this technology, thereby minimizing exposure to ionizing radiation.
References
- Pedrosa I, Lafornara M, Pandharipande PV, et al. Pregnant patients suspected of having acute ppendicitis: Effect of MR imaging on negative laparotomy rate and appendiceal perforation rate. Radiology. 2009;250:749-757.
- Pedrosa I, Zeikus EA, Levine D, Rofsky NM. MR imaging of acute right lower quadrant pain in pregnant and nonpregnant patients. RadioGraphics. 2007;27:721-743.
- Pedrosa I, Levine D, Eyvazzadeh AD, et al. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006;238:891-899.
- Oto A. MR imaging evaluation of acute abdominal pain during pregnancy. Magn Reson Imaging Clin N Am. 2006;14:489-501, vi.
- Oto A, Ernst RD, Ghulmiyyah LM, et al. MR imaging in the triage of pregnant patients with acute abdominal and pelvic pain. Abdom Imaging. 2009;34:243-250.
- Brown M, Birchard KR, Semelka RC. Magnetic resonance evaluation of pregnant patients with acute abdomial pain. Ultrasound CT MRI. 2005;26:206-211.
- Wiskirchen J, Groenewaeller EF, Kehlbach R, et al. Long-term effects of repetitive exposure to a static magnetic field (1.5 T) on proliferation of human fetal lung fibroblasts. Magn Reson Med. 1999;41:464-468.
- Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188:1447-1474.
- Glover P, Hykin J, Gowland P, et al. An assessment of the intrauterine sound intensity level during obstetric echo-planar magnetic resonance imaging. Br J Radiol. 1995;68:1090-1094.
- Vadeyar S, Moore RJ, Strachan BK, et al. Effect of fetal magnetic resonance imaging on fetal heart rate patterns. AM J Obstet Gynecol. 2000;182:666-669.
- Baker P, Johnson IR, Harvey PR, et al. A three-year follow-up of children imaged in utero with echo-planar magnetic resonance. AM J Obstet Gynecol. 1994;170:32-33.
- Clements H, Duncan KR, Fielding K, et al. Infants exposed to MRI in utero have a normal paediatric assessment at 9 months of age. Br J Radiol. 2000;73:190-194.
- Long SS, Long C, Lai H, Macura KJ. Imaging strategies for right lower quadrant pain in pregnancy. AJR Am J Roentgenol. 2011;196:4-12.
- Rofsky NM, Pizzarello DJ, Duhaney MO, et al. Effect of magnetic resonance exposure combined with gadopentetate dimeglumine on chromosomes in animal specimens. Acad Radiol. 1995;2:492-496.
- Kanal E. Pregnancy and the safety of magnetic resonance imaging. Magn Reson Imaging Clin N Am. 1994;2:309-317.
- Romine L, Reena C, Katarina K, et al. Pregnancy and fetus. In: Semelka R, ed. Abdominal-Pelvic MRI, 3 ed. Hoboken, N.J: Wiley-Blackwell, 2010: 1559-1636.
- Tyndall D, Sulik KK. Effects of magnetic resonance imaging on eye development in the C57BL/6J mouse. Teratology. 1991;43:264-275.
- Panigel M, Wolf G, Zeleznick A. Magnetic resonance imaging of the placenta in rhesus monkeys, Macaca mulatta. J Med Primatol. 1998;17:3-18.
- Hutcheson DP, Gray DH, Venugopal B, Luckey TD. Safety of heavy metals as nutritional markers. Environ Qual Saf Suppl. 1975;1:74-80, 114-115.
- Inci E, Kilickesmez O, Hocaoglu E, et al. Utility of diffusion-weighted imaging in the diagnosis of acute appendicitis. Eur Radiol . 2011;21:768-775.
- Singh AK, Desai H, Novelline RA. Emergency MRI of acute pelvic pain: MR protocol with no oral contrast. Emerg Radiol. 2009;16:133-141.
- Birchard KR, Brown MA, Hyslop WB, et al. MRI of acute abdominal and pelvic pain in pregnant patients. AJR Am J Roentgenol . 2005;184:452-458.
- Low RN, Francis IR. MR imaging of the gastrointestinal tract with i.v., gadolinium and diluted barium oral contrast media compared with unenhanced MR imaging and CT. AJR Am J Roentgenol. 1997;169:1051-1059.
- Hirohashi S, Hirohashi R, Uchida H, et al. MR cholangiopancreatography and MR urography: Improved enhancement with a negative oral contrast agent. Radiology. 1997;203:281-285.
- Cronin CG, Lohan DG, Browne AM, et al. Does MRI with oral contrast medium allow single-study depiction of inflammatory bowel disease enteritis and colitis? Eur J Radiol. 2010;20:1667-1674.
- Siddiki H, Fidler J. MR imaging of the small bowel in Crohn’s disease. Eur J Radiol. 2009;69:409-417.
- McGory ML, Zingmond DS, Tillou A, et al. Negative appendectomy in pregnant women is associated with a substantial risk of fetal loss. J Am Coll Surg. 2007;205:534-540.
- Andersen B, Nielsen TF. Appendicitis in pregnancy: Diagnosis, management and complications. Acta Obstet Gynecol Scand. 1999;78:758-762.
- Lee K, Rofsky N, Pedrosa I. Localization of the appendix at MR imaging during pregnancy: Utility of the cecal tilt angle. Radiology. 2008;249:134-141.
- Mazziotti S, Costa C, Ascenti G, et al. MR cholangiopancreatography diagnosis of juxtapapillary duodenal diverticulum simulating a cystic lesion of the pancreas: Usefulness of an oral negative contrast agent. AJR Am J Roentgenol. 2005;185:432-435.
- Petersein J, Reisinger W, Mutze S, Hamm B. Value of negative oral contrast media in MR cholangiopancreatography (MRCP). Rofo. 2000;172:55-60.
- Sugita R, Nomiya T. Disappearance of the common bile duct signal caused by oral negative contrast agent on MR cholangiopancreatography. J Comput Assist Tomogr. 2002;26:448-450.
- Semelka R. Abdominal-Pelvic MRI, 3rd ed. Hoboken, NJ: Wiley-Blackwell, 2010.
- Moschos E, Sreenarasimhaiah S, Twickler DM. First-trimester diagnosis of cesarean scar ectopic pregnancy. J Clin Ultrasound. 2008;36:504-505.
Related Articles
Citation
MR imaging of abdominal and pelvic pain in pregnancy. Appl Radiol.
August 28, 2012