Magnetic resonance techniques in lymph node imaging
Images


Dr. Shetty is a Resident in Radiology and Dr. Harisinghani is an Assistant Professor and a Co-Director of Abdominal MR in the Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Superior soft-tissue contrast and resolution has made magnetic resonance imaging (MRI) an important tool in the armamentarium of the oncologic imager, providing staging information that predicts prognosis, guides selection of therapy, and evaluates response to treatment. Several modalities have been employed to attempt accurate assessment of tumor stage (T stage), nodal status (N stage), and the existence of distant metastasis (M stage), including computed tomography (CT), MRI, and positron emission tomography (PET). Each modality has its own strengths and weaknesses: thanks to excellent soft-tissue contrast and resolution, MRI has been particularly useful in the evaluation of the primary tumor and detection of distant metastasis.
This review details the historic challenges and current approaches to the application of MRI to the third domain of oncologic imaging, lymph nodes. MRI has traditionally relied on size criteria and morphology as a predictor of malignancy in lymph nodes, limiting accuracy of the modality and potentially providing inaccurate staging information. This understaging of nodal status has important clinical implications, because failure to diagnose nodal metastasis may prevent a patient from receiving appropriate or even curative treatment. Similarly, there are also implications of misdiagnosing metastasis in a normal lymph node, as reduced specificity for nodal metastasis will result in unnecessary treatment or exclusion of treatment options. Surgical methods, even less invasive methods such as laparoscopy, confer procedural risk and morbidity. While considered the gold standard, surgical exploration itself suffers from false-negative findings resulting from the fallibility of intraoperative frozen section. 1
This article will review the traditional methods of lymph node characterization by MRI and will discuss newer imaging approaches that attempt to address the modality's historic shortcomings; these approaches include the evaluation of signal intensity, dynamic gadolinium contrast enhancement, the use of ultrasmall superparamagnetic iron oxides (USPIO), MR spectroscopy, and interstitial application of contrast. Particular attention will be paid to the mechanisms and oncologic applications of USPIO (also known as lymphotrophic superparamagnetic nanoparticles), a contrast agent with exciting potential supported by a growing body of clinical literature.
Size and morphologic criteria
The traditional approach to MRI of lymph nodes has relied on size criteria to distinguish metastatic from uninvolved nodes. The efficacy of this approach depends heavily on the selection of a threshold size, necessitating a tradeoff between setting a low size threshold (highly sensitive but poorly specific) and a high size threshold (increased specificity at a cost of diminished sensitivity). A range of acceptable threshold sizes has been proposed, which vary in the use of different aspects of nodal measurement, such as long-axis or short-axis diameter, and the application to specific nodal groups. 2-4 Studies using sizes derived from imaging 5-11 and gross specimens 12-14 show that although size criteria can be applied with some success, the approach frequently overlooks metastasis, particularly when the metastasis involves only microscopic or partial infiltration of the lymph node. The specificity of size criteria also deteriorates because of benign inflammatory or infectious lymph node enlargement, leading to incorrect characterization of a benign lymph node as malignant. MR is no different 15 or slightly worse, 5 basing the judgment on size criteria alone, compared with CT in the assessment of regional lymph node metastasis.
The addition of morphologic criteria to the evaluation of lymph nodes seeks to exploit changes to the normal ovoid lymph node shape that arise from tumor infiltration. These changes could include either a more rounded shape, in which the long-to-short axis ratio decreases, or eccentric cortical hypertrophy. A commonly used size threshold in the pelvis accounts for this change in morphology, using 10 mm in short axis diameter for ovoid lymph nodes, while using a smaller threshold (8 mm) as a cutoff in rounded lymph nodes. 3 In a study of 4043 axillary lymph nodes in the setting of breast cancer, 16 the use of either eccentric cortical hypertrophy or a long axis diameter >10 mm plus a long-to-short axis ratio of <1.6 resulted in a sensitivity of 79% and a specificity of 93% for the detection of lymph node metastasis, with nearly all false-negative findings in the axillae showing metastatic lymph nodes measuring <10 mm.
Application of these size criteria requires detection of lymph nodes, a task that is complicated by motion, the presence of adjacent structures, and limitations in resolution and signal-to-noise ratio. Using a three-dimensional (3D) magnetization-prepared rapid gradient-echo (MPRAGE) T1-weighted sequence, Jager et al 3 reported a sensitivity and specificity of 75% and 98%, re-spectively, for lymph node metastasis in patients with prostate and bladder cancer. However, because their method was dependent on a size threshold for nodal characterization, they failed to detect microscopic metastases in 11 of 134 patients. Using a 3D-fast low-angle shot (FLASH) sequence performed after bolus injection of gadolinium, Hasegawa et al 17 reported 92% sensitivity and 78% specificity for the detection for hilar lymph node enlargement, results similar to those from CT and PET techniques. Continued evolution in MR hardware and development of innovative pulse sequences will improve detection of lymph nodes in increasingly efficient ways; however, even improved detection may not be sufficient to optimize the performance of MRI without a better means of lymph node characterization.
T2 signal intensity
The improved soft-tissue contrast and fluid sensitivity of MRI suggest an additional approach to the evaluation of lymph nodes for metastasis: using signal characteristics of lymph nodes in the absence of contrast media as a means of differentiating benign from malignant. However, results with these techniques have been mixed. Brown et al 10 evaluated 437 lymph nodes with high-resolution MR techniques, using a T2-weight-ed fast spin-echo (FSE) sequence with a relatively long acquisition time that took advantage of 4 averaged signals to maintain signal despite the high resolution. They saw no significant difference in size between benign and malignant lymph nodes and achieved a sensitivity of 81% and a specificity of 68% with a size threshold of 5 mm. Adding evaluation of heterogeneous nodal signal intensity or an irregular border improved these parameters such that the sensitivity and specificity for nodal metastasis were 85% and 97%, respectively. However, these results excluded lymph nodes <3 mm and metastatic lymph nodes outside of the field-of-view, a particular concern given the high-resolution techniques employed.
In a study evaluating 140 mediastinal lymph nodes in patients with non-small-cell lung cancer, 18 the use of a T2-weighted respiratory-trig-gered short tau inversion recovery (STIR) turbo spin-echo (TSE) sequence allowed differentiation of metastatic lymph nodes with a sensitivity, specificity, and accuracy of 100%, 96%, and 96%, respectively; this technique relied on comparing the signal intensity of a lymph node with a 0.9% saline phantom and compared favorably with T1-weighted spin-echo (SE) imaging and CT (Figure 1).
The presence or absence of central necrosis is another predictive feature of malignancy that can be exploited in the analysis of lymph node metastasis. In a study of 949 lymph nodes in 43 women with cervical carcinoma, 19 MRI had a sensitivity and specificity of 70.6% and 89.8%, respectively, using a size threshold of 10 mm in long-axis diameter or the presence of central necrosis. Similarly, the presence of central necrosis is the single most accurate indicator of malignancy in the head and neck in the presence of squamous cell carcinoma. 20,21 However, the presence of central necrosis is very nonspecific when applied to other areas of the body, such as the mesentery, retroperitoneum, and pelvis, where infection or inflammation can result in a similar appearance. 22
Dynamic gadolinium enhancement
Several investigators have attempted to use the enhancement characteristics of lymph nodes after bolus administration of intravenous gadolinium as a discriminant between malignant and benign lymph nodes. Simple comparison of signal intensity after intravenous gadolinium administration has not been effective in differentiating benign from malignant lymph nodes. 23 A more complex approach relies on a dynamic analysis of enhancement kinetics, based on alterations in tumor microcirculation: flow characteristics and blood volume, microvascular permeability, and increased fractional volume of the extravascular extracellular space. 24 In a study of mediastinal lymph nodes in 9 patients with bronchogenic carcinoma, Laissy et al 25 found peak enhancement in metastatic lymph nodes within 60 to 80 seconds after gadolinium enhancement, with a slow washout thereafter. In contrast, reactive lymph nodes showed gradual increase in contrast enhancement without a peak value in the first 6 to 8 minutes.
Using a T1-weighted 3D FLASH sequence with a 44-second acquisition time, Murray et al 26 compared axillary lymph node enhancement to adjacent fat; it was concluded that using the presence of at least 1 lymph node with an enhancement index of >21% and nodal area of >0.4 cm 2 would allow discrimination of patients with axillary lymph node metastasis with a sensitivity of 100% and specificity of 56%. The relatively low specificity was deemed acceptable, because the high sensitivity for metastasis would ensure that all patients with metastasis would undergo surgical axillary lymph node dissection. Using a threshold of more than 100% increase in signal intensity in axillary lymph nodes on initial postcontrast images in 65 patients with invasive breast cancer, Kvistad and colleagues 27 showed an 83% sensitivity and a 90% specificity for correct diagnosis of axillary lymph node metastases per patient. Interestingly, their results showed no improvement in accuracy when additional size or appearance characteristics were added to the evaluation. However, a limitation of both studies was that a node-by-node correlation was not performed, raising the possibility that the abnormalities seen on MR did not correlate specifically with foci of metastasis.
Fischbein and colleages 24 evaluated squamous cell cancer of the head and neck using a two-dimensional fast spoiled gradient-recalled sequence after a single bolus of intravenous gadolinium. Their results are almost opposite from those achieved in other neoplasms: correlating enhancement characteristics with pathologic specimens showed significantly longer time to peak enhancement, lower peak enhancement, lower maximum slope, and slower washout of contrast material in metastatic lymph nodes (Figure 2). Of note, the technical constraints of this dynamic imaging, as well as artifacts related to motion, precluded complete coverage of the entire area of interest and limited the radiologic evaluation to 68 of the 129 pathologically identified lymph nodes. It was hypothesized that, in the specific case of squamous cell carcinoma, tumor tissue may actually have decreased blood flow relative to normal or hyperplastic lymphoid tissue and that squamous cell carcinoma of the head and neck may not have increased microvessel density.
Ultrasmall superparamagnetic iron oxide particles
A particularly promising technique for the evaluation of lymph nodes in the setting of malignancy relies on the use of USPIO particles. 28,29 These particles were developed as an alternative to larger superparamagnetic iron oxide particles (SPIO), which are rapidly cleared by the mononuclear phagocytic systems of the liver and spleen, allowing little uptake in other tissues. Ultrasmall superparamagnetic iron oxide particles have a longer blood half-life and accumulate in the normal reticuloendothelial structure of lymph nodes, providing a means to distinguish malignant and benign lymph nodes without reliance solely on size or morphologic criteria. 30
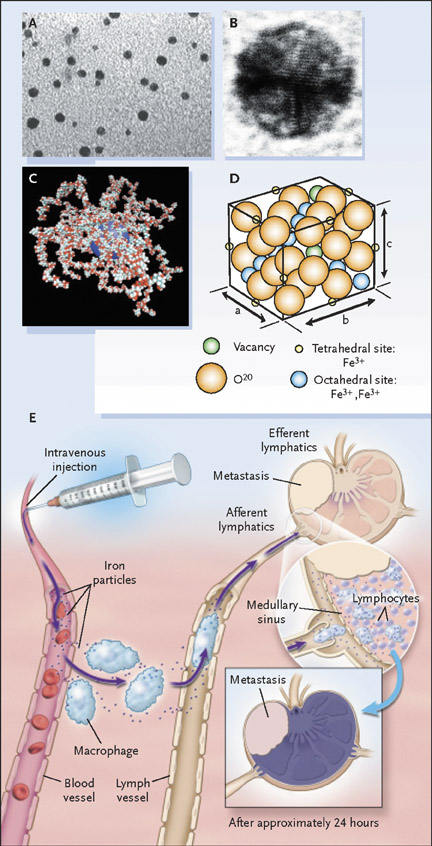
Ultrasmall superparamagnetic iron oxide particles belong to a larger family of the SPIO, in which particle size influences very different chemical and kinetic properties and therefore produces different clinical applications. 31 Each bio degradable particle of the USPIO ferumoxtran-10 (AMI-227; Combidex, Advanced Magnetics, Cambridge, MA; Sinerem, Laboratoire Guerbet, Aulnay-sous-Bois, France) is composed of a monocrystalline, inverse spinel, SPIO core (2 to 3 nm 32 or 4.3 to 6.0 nm 33 ) coated with polymers (low molecular weight dextran) to prevent uncontrolled aggregation. The method of particle preparation determines the final mean particle size (approximately 17 to 21 nm 34 or 20 to 40 nm 31,33 ) and composition 22 (Figures 3A through 3D). The agent is provided as a lyophilized powder that is reconstituted and administered over approximately 30 minutes in an intravenous dose of 2.6 mg/kg, an amount that has been found to optimize signal decrease in normal lymph nodes. 35 Clinical trials have documented the safety of this agent, 34-36 with the most common side effect being back pain, occurring in about 3% to 6% of patients; this is of uncertain cause and usually resolves with temporary cessation of the infusion. Other less commonly reported minor side effects are rash, transient mild hypotension, and headache. 22,34,37
After administration of contrast material, the agent is distributed into lymph nodes throughout the body and is usually imaged 24 hours later. The contrast agent distributes symmetrically throughout the body after intravenous administration, which aids in a comprehensive nodal evaluation that does not depend on the site of injection. 38 Entrance into lymph nodes is via 2 mechanisms: first, direct transcapillary passage from venules into the medullary sinuses of lymph nodes and, second, nonselective endothelial transcytosis into the interstitial space, from where the particles drain into lymph nodes via the lymphatic system. 28,29,39 Once within the lymph node, the particles are phagocytosed and subsequently accumulate within macrophages (Figure 3E). This accumulation of the USPIO particles has 2 major effects: a predominant susceptibility effect, as well as T2 shortening, resulting in decreased signal on T2- and T2*-weighted images. 40 The susceptibility effect is most important, with microscopic field gradients that lead to diffusion and loss of phase coherence. There is also a T2-shortening effect caused by local field inhomogeneities that promotes transverse relaxation.
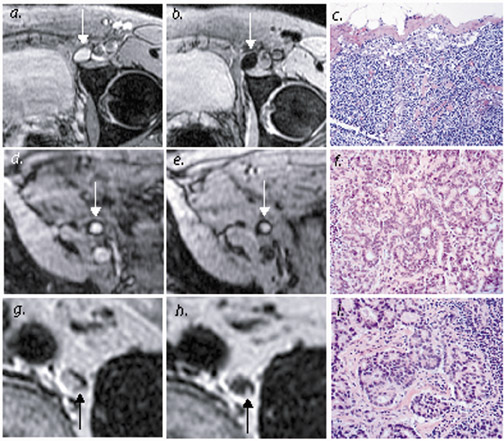
The end result is that USPIO is a "negative" contrast agent, one which is taken up by benign lymph nodes with preserved nodal architecture; this "negative enhancement" appears as decreased signal intensity on T2- and T2*-weighted images. 36,41 This accumulation of USPIO in these normal lymph nodes corresponds to the macrophages in the medullary sinuses rather than the lymphocyte-rich follicles of the lymph nodes, as shown on 9.4T imaging. 30 In contrast, areas of metastatic nodal infiltration lack reticuloendothelial structure and macrophages and therefore do not accumulate USPIO, resulting in a lack of uptake in all or part of a malignant lymph node 36 (Figure 4). Metastasis is therefore identified in lymph nodes that are either entirely or partially unchanged in signal intensity on T2- and T2*-weighted scans.
Analysis of USPIO-enhanced MR is most often performed with direct comparison of scans obtained before and after USPIO administration. While both T2- and T2*-weighted images show de-creased signal in benign lymph nodes, T2-weighted fast spin-echo techniques are generally favored for their superior resolution, despite the increased sensi-tivity to susceptibility achieved on gradient-echo sequences. In fact, the "blooming artifact" due to susceptibility on gradient-recalled echo (GRE) can actually hinder analysis, resulting in overestimated lymph node size and obscured areas of micrometastasis (which are surrounded by UPSIO-enhanced normal lymph node tissue). 42,43 Quantitative determination of changes in T2* from dual echo time (TE) images in lymph nodes may permit a more objective analysis of signal changes with significant differences seen between benign and malignant lymph nodes, even in the case of partial infiltration; this may have future application in partial automation of image analysis. 44 In lower concentrations, USPIO agents also cause T1- shortening, manifesting as increased signal on T1-weighted sequences; this effect has been shown in neoplasms with associated leakage of USPIO particles into the interstitium. 40
Interpretation of USPIO-enhanced images requires careful attention to MRI technique and experience in identification of even small lymph nodes. The results of USPIO-enhanced MRI have also been used successfully to direct image-guided lymph node biopsy, allowing pathologic correlation without a more invasive surgical procedure. 45
Several patterns of USPIO uptake have been identified. Malignant patterns in-clude complete lack of enhancement with USPIO, heterogeneous enhancement, discrete focal defects (representing small focal metastatic deposits), and peripheral signal loss with maintained signal centrally (in the absence of a fatty hilum). 32,34 False-positive results can be generated by focal nodal lipomatosis or prominent fatty hila, 22 and inclusion of T1-weighted images may help reduce these false-positive interpretations by increasing conspicuity and recognition of fat. 46 False-negative results most commonly relate to the presence of micro-metastasis below the spatial resolution of the imaging sequence. False-positive results have been seen in benign lymph nodes with reactive lymphoid follicular hyperplasia; the relative scarcity of macrophages results in decreased USPIO uptake and incorrect categorization of lymph nodes as malignant. 36 Similarly, granulomatous disease or other infection can reduce phagocytic activity and reduce uptake of UPSIO in normal nodes, a particular problem in the chest and mediastinum. 47 Heterogeneous uptake in normal lymph nodes may relate to a heterogeneous distribution of macrophages 30 or to areas of focal lymphoid hyperplasia. 48
Uptake of USPIO agents is not limited to the lymph nodes alone: decreases in signal intensity on T2- and T2*-weighted images have been seen in liver, spleen, bone marrow, and kidneys after USPIO administration. The long half-life of USPIO agents in the vascular system has prompted use of the agent for MR angiography; this application depends on the long imaging window and the T1-shortening effects of the contrast agent. 22
The use of USPIO agents for lymph node applications requires evaluation in the context of particular primary neoplasms and body regions, because the challenges encountered in the MR imaging of various body parts are unique. Direct comparison of the many clinical trials of USPIO is hindered by marked variations in MR technique and different methods of statistical analysis. Differences in MR technique and operator experience alter the ability to detect and analyze signal changes in lymph nodes. Published statistics are usually based only upon nodes with radiologic and pathologic correlation, often excluding very small nodes (1 to 3 mm) that are seen at pathologic analysis only; this precludes direct comparison with gold standard surgical techniques and somewhat undermines the confidence in staging based only on noninvasive methods. These studies also use different benchmarks, including performing analyses at a patient, nodal group, or individual lymph node level, complicating direct comparisons. Despite these limitations, however, a growing body of literature allows us to evaluate trends in the results of USPIO-enhanced MR and to highlight the promise of the technique.
The largest single trial evaluating ferumoxtran-10 to date is a phase III trial of Combidex, which evaluated 152 patients with primary neoplasms of the head and neck, breast, chest, abdomen, and pelvis. 34 Using a node-by-node analysis with histopathologic correlation, the sensitivity, specificity, and accuracy of USPIO-enhanced MR was 83%, 77%, and 80%, respectively, using both pre- and postcontrast images and 85%, 85%, and 85%, respectively, with the postcontrast images alone. This study highlighted one of the major benefits of USPIO relative to traditional size criteria: the potential for USPIO to reveal micrometastasis that does not grossly alter the size or shape of the metastatic lymph node. 34
Ultrasmall superparamagnetic iron oxide agents have been particularly successful in the analysis of the head and neck, where there is a complex distribution of normally visualized lymph nodes; previous studies have shown poor performance of traditional size criteria on both MR and 5,8 An accurate noninvasive method of nodal assessment is particularly important because of the morbidity and potential cosmetic deformity conferred by a surgical procedure. The use of USPIO-enhanced MR for differentiation of benign and malignant lymph nodes in the head and neck has revealed a range of sensitivities between 84% and 95% and a range of specificities between 77% and 97%. 34,41,49-51 Patients with neoplasms of the head and neck were the most accurately assessed subset of patients in the larger phase III trial of Combidex. 34 The low end of the range in sensitivity (77%) is based on a European phase III trial of Sinerem focusing on 81 patients with head and neck malignancies 51 and has been partially attributed to higher rates of artifact related to patient motion and susceptibility; in this trial, specificity was improved with USPIO, while no significant improvement in sensitivity was seen compared with precontrast imaging (which showed an unusually high sensitivity). A smaller study of USPIO in head and neck cancer showed that USPIO-enhanced MRI resulted in changed surgical management in 7 of 27 patients and a correct diagnosis of metastatic nodal level in 26 of 27 patients. 52
The performance of UPSIO-enhanced MRI in the evaluation of the axilla in patients with breast cancer has also been favorable; initial reported sensitivities of USPIO-enhanced MR (in 9 patients 53 and 20 patients 54 ) have ranged between 73% to 83% and specificities have ranged between 92% to 97% for the detection of lymph node metastasis. 53,54 Specific correlation of imaging to pathologic features was not performed in these cases due to the absence of anatomic landmarks in the axilla. When analyzed at a patient level, the sensitivity and specificity for detection of nodal metastasis in one study increased to 82% and 100%, respectively. 54 Looking at the subset of patients with breast cancer from the larger phase III trial, USPIO-enhanced MR had a sensitivity of 83%, a specificity of 78%, and an accuracy of 80%. 34 More recent work has documented improved performance of the technique: 25 patients with breast cancer were enrolled in another phase III trial, in which node-by-node pathologic correlation was achieved in 136 lymph nodes. 55 In this group, USPIO-enhanced MR resulted in a sensitivity, specificity, and accuracy of 92%, 99%, and 98%, respectively, for detection of nodal metastasis, improving on traditional MR size criteria, which had a sensitivity and specificity, respectively, of 58% and 56%. 55
In non-small-cell cancer, PET and mediastinoscopy have traditionally been used to stage mediastinal lymph nodes, an important determinant in clinical staging and the differentiation between resectable versus unresectable disease. The chest is a particularly challenging location for the application of UPSIO-enhanced MR, given the high prevalence of benign lymph node enlargement and the specific challenges of MR imaging that include motion and susceptibility artifact from air-soft tissue interfaces. An investigation of USPIO-enhanced MR in 18 patients with known lung cancer showed a sensitivity of 92% and a specificity of 80%. 56 A study of 12 patients, 6 of whom had cancer, revealed that USPIO-enhanced MR had a sensitivity of 100% but a specificity of only 37.5%. 47 The high rate of false-positive results was attributed to the prevalence of granulomatous infection in the mediastinum, leading to decreased USPIO uptake in benign lymph nodes. One study comparing PET with CT included a small sample of USPIO-enhanced MR 57 (9 patients) and showed an overall sensitivity and specificity of 86% and 82%, respectively; this was not significantly different from the results obtained with PET in the entire study population (64 patients), which had a sensitivity and specificity of 70% and 86%. A low specificity of UPSIO in the chest and mediastinum was also seen in the phase III study of Combidex, presumably also because of the higher prevalence of granulomatous infection in the mediastinum; use of the approach in this population for detection of nodal metastasis showed a sensitivity, specificity, and accuracy of 89%, 47%, and 63%, respectively. 34
The use of USPIO-enhanced MR in the abdomen and pelvis has been quite successful; the improved differentiation of benign versus malignant lymph nodes plays an important role in staging and surgical planning in this population. Initial experience in this area correlated partial uptake of USPIO to partial metastatic nodal infiltration 58 and revealed a subset of lymph nodes with increased signal on T1-weighted images due to the T1-shortening effects of USPIO and increased capillary permeability. 36 Early experience in 2 studies, which enrolled 3036 and 1958 patients with a variety of abdominal and pelvic malignancies, showed sensitivities of 100% and 93% and specificities of 80% and 100%, respectively. This early experience showed the possibility of detecting partial metastatic infiltration in lymph nodes: in one study, the false-negative signal drop in 2 malignant lymph nodes was deemed a subjectively different, more heterogeneous pattern that correlated with heterogeneous lymph node infiltration. 58 At the same time, in the other study, 20 of 80 lymph nodes available at pathologic analysis were not seen at MRI, either due to small size or partial volume effects, and, of these, nearly a third harbored metastasis. 36 A study of 39 patients with gynecologic malignancies showed an improvement of sensitivity from 53% to 86% to 88% without loss of specificity in metastatic lymph node detection using USPIO. 46 A more recent, smaller study of 18 patients with testicular cancer also showed the value of USPIO-enhanced MR, which showed a sensitivity, specificity, and accuracy of 86.6%, 96.9%, and 95.8%, respectively, in the detection of lymph node metastasis. The use of USPIO in patients with malignancies of the abdomen and pelvis in the larger phase III clinical trial resulted in a sensitivity, specificity, and accuracy of 80%, 83%, and 81%, respectively. 34
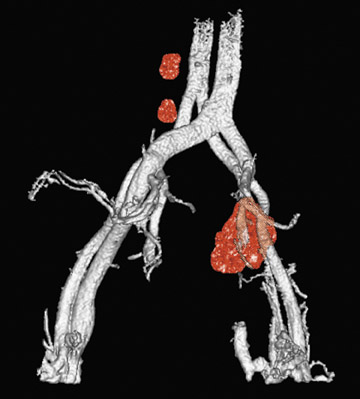
Harisinghani and colleagues 32 reported on a large population (80 patients) with prostate cancer, where USPIO-enhanced MRI significantly increased sensitivity for detection of lymph nodes, from 35.4% to 90.5%. Specificity was also increased, from 90.4% to 97.8%. These results were particularly notable for the 45 of 63 metastatic lymph nodes that did not achieve traditional size criteria for malignancy but which were identified with USPIO. Careful attention to lymph node identification relative to anatomic landmarks allowed precise correlation with MR images (Figure 4 and Figure 5).
In summary, the benefits of USPIO-enhanced MR imaging seem to be greatest with certain areas of the body, including the head and neck, axilla, retroperitoneum, and pelvis. Evaluation of mediastinal lymph nodes suffers from the prevalence of granulomatous and other infections, decreasing specificity of the UPSIO-enhanced technique, as well as from technical limitations imposed by motion and susceptibility. Several areas of investigation are critical: correlation of USPIO staging results with outcomes and treatment, evaluation of the cost effectiveness of the technique particularly given the logistical considerations of a 24-hour delay between contrast administration and imaging, and specific delineation of methodologies and MRI protocols for optimal use. Increasing clinical experience will permit more accurate comparison of USPIO approaches as applied to different neoplasms and clinical situations. Continued improvements in MR imaging will also further improve the technique, particularly through improved spatial and contrast resolution that will allow identification of smaller lymph nodes and micro-metastases without overly detrimental loss in coverage or noise.
MR spectroscopy
Applying the techniques of MR spectroscopy (MRS) to the evaluation of lymph nodes is another possible approach to differentiation of benign versus malignant lymph nodes; similar to USPIO and dynamic gadolinium approaches, the technique attempts to extend MRI beyond simple anatomic mapping. In a study of 39 patients with a combination of ductal carcinoma in situ and invasive ductal carcinoma, 59 axillary lymph node MRS had a sensitivity of 82% and specificity of 100% (correlating with pathologic results obtained from ultrasound-guided lymph node biopsy). These results were based on the presence or absence of choline (at 3.2 ppm). Of note, the targeted lymph nodes ranged in diameter between 1 and 5 cm, and 2 patients with negative biopsies and MRS were subsequently found to have metastasis in small axillary nodes measuring 4 mm, too small to be targeted by either method. A preliminary study in cervical lymph node metastases showed abnormal MR spectra, including elevation in choline to creatine ratio and elevated lactate, when compared with normal muscle tissue. 60
Interstitial contrast administration
The interstitial administration of MR lymphangiographic contrast agents represents a different approach to visualization of the lymphatic system on MR. The enhancement of the lymphatic system (including both lymphatic vessels and nodes) permits identification of the drainage pathway and possibly a sentinel lymph node that can be targeted for biopsy. Alterations or abnormalities in lymphatic flow can also be used to directly diagnose lymphatic metastasis, in a manner similar to conventional lymphangiography. However, these techniques sacrifice the more global lymph node evaluation afforded by, for example, intravenous administration of USPIO agents. The technique is also susceptible to concerns that arise from conventional interstitial sentinel node detection techniques, including radiolabeled sulfur colloid and methylene blue, in that the sites of injection may not truly reflect all potential drainage pathways of the primary tumor under study. A benefit of these agents is that they work primarily to promote T1-shorten-ing and therefore increase signal intensity on T1-weighted sequences, creating "positive contrast" images of lymph nodes and lymphatic vessels. There are also potential benefits of lower contrast dose and reduced systemic side effects of contrast. Preliminary work has shown experimental success of this technique with several agents, including MS-325 (a paramagnetic agent with novel chemical groups that promote reversible binding to albumin, increasing intravascular half-life), 61 conventional gadolinium agents, 62-64 and macromolecular gadolinium polymers and aggregates 65-70 (Figure 6).
Conclusion
The field of MR oncologic imaging represents an exciting application for current imaging techniques, offering important information with definite clinical implications, including prognosis and treatment selection. The traditional strengths of MRI, with improved soft-tissue contrast and delineation of tissue planes, are related to staging of the primary tumor or detection of metastasis. Extending its value as a modality for the evaluation of nodal status may increase accuracy of staging or even allow for a single imaging evaluation that includes the primary tumor and adjacent lymph nodes. As detailed in this review, several approaches have been developed that seek to improve on the results obtained with traditional size criteria alone; each of these has shown promising results in attempts to image the function and physiology of lymph nodes to increase accuracy. Unfortunately, direct comparison between different approaches (or even between different studies using the same approach, as in the case of USPIO) is hindered by marked differences in technique and methods of analysis employed in various investigations. As the experience with each approach grows, it will be easier to draw more accurate direct comparisons and identify the appropriate role of each. With inevitable continued technologic improvements, lymph node imaging will no doubt continue to represent an exciting frontier in MR, with the potential to have a large impact on future clinical practice.