Magnetic resonance imaging of the pancreas
Images




Dr. Oliva is a Clinical Fellow, Division of Abdominal Imaging and Intervention, Department of Radiology, Brigham and Women's Hospital-Harvard Medical School, Boston, MA. Dr. Mortelé is the Associate Director, Division of Abdominal Imaging and Intervention, and the Director, Abdominal and Pelvic MRI, Brigham and Women's Hospital, and an Associate Professor of Radiology, Harvard Medical School. Dr. Erturk is a Research Fellow, Division of Abdominal Imaging and Intervention, Brigham and Women's Hospital-Harvard Medical School. Dr. Ros is a Professor of Radiology, Harvard Medical School; and the Executive Vice Chairman and Associate Radiologist-in-Chief, Department of Radiology, Brigham and Women's Hospital.
Currently, magnetic resonance imaging (MRI) plays a secondary role in the diagnosis and imaging workup of patients with pancreatic diseases, when compared with multidetector-row computed tomography (MDCT). However, technical innovations in MRI, such as the development of phased-array multicoils, enhanced gradients, and methods to reduce motion-related artifacts, allow us to obtain images of the pancreas with excellent contrast resolution in a reasonable examination time. Furthermore, the evaluation of the pancreas can be optimized by the use of MR pancreatography (MRP), which depicts the pancreatic ductal system, and with MR angiography (MRA), which visualizes the peripancreatic vessels. At present, this "all-in-one" approach, combining pancreatic parenchymal MRI, MRP, and MRA, is presumably the most cost-effective imaging technique in the evaluation of pancreatic diseases.
This article addresses the current techniques, the main indications, and the imaging features of common pancreatic diseases using state-of-the-art MRI.
Technique
To evaluate pancreatic lesions accurately, the use of multiple pulse sequences that provide complementary information is required, and, in general, a combination of T1-weighted (T1W) and T2-weighted (T2W) sequences is obtained. In the ideal MRI sequence, the spatial resolution is maximized within a short acquisition time while the signal-to-noise ratio is maintained sufficiently high. The development of surface coils oriented to image a specific volume of the body (phased-array surface multicoil system or torso coil) proved to be an important tool that improves the diagnostic value of the individual pulse sequences and produces higher-resolution images compared with the whole-volume body coil. 1
T1W imaging
T1-weighted imaging that uses conventional spin-echo (SE) technique requires significant imaging time and is no longer routinely performed. 2 Instead, we use breath-hold T1W spoiled gradient recalled echo (GRE) imaging. 3,4 These sequences generate an echo by gradient reversal, allowing a short echo time (TE) and, consequently, a much faster sequence. 2 For these, the flip angle should be ≥70˚, which is adequate to obtain appropriate T1 contrast (demonstrated by higher signal intensity from the liver than from the spleen); the echo delay time should be <5 msec; and the repetition time (TR) should be sufficient to image the entire pancreas in a multislice acquisition (usually >100 msec). 5 These spoiled gradient images (eg, fast low-angle shot [FLASH], multiplanar gradient-recalled [MPGR]) can be obtained with and without fat suppression. 6
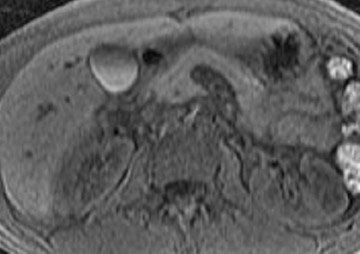
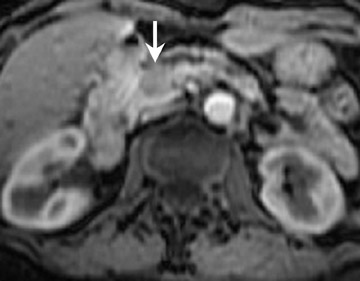
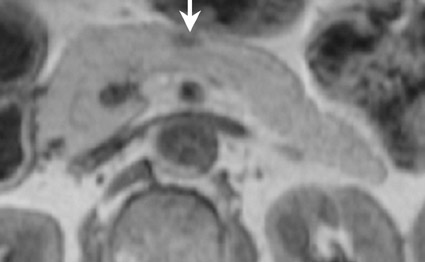
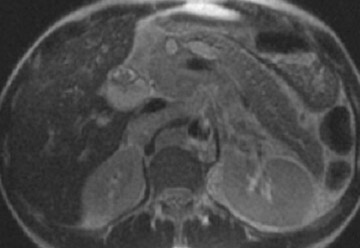
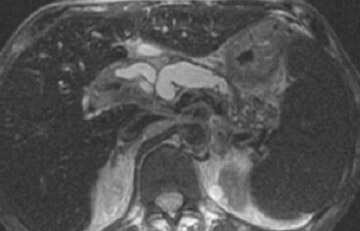
The normal pancreas has the highest signal intensity of the intra-abdominal organs on fat-suppressed T1W images (Figure 1). This is attributed to the larger amount of aqueous protein within the glandular elements, an abundance of endoplasmatic reticulum of the protein-producing acinar cells, and the high content of paramagnetic ions, such as manganese, within the pancreas. 7 In elderly patients, this high T1 signal intensity may be reduced in comparison with the signal intensity of the liver, most likely because of age-related fibrosis. 8 The most common pancreatic diseases (ie, pancreatitis and pancreatic adenocarcinoma) have longer T1 because of increased free water protons and, therefore, have lower signal intensity compared with that of the surrounding normal pancreatic parenchyma. 9 It has been shown that the conspicuity of pancreatic tumors and the delineation of the pancreas from surrounding fat are best established on fat-suppressed T1W images. 9,10
T2W imaging
T2-weighted imaging is performed with a longer TR and TE (ie, TR >1800 msec, and at least one echo with a TE ≥60 msec) when compared with T1W sequences. 9,10 T2 images are achieved either by conventional SE, fast SE, or GRE imaging. Images obtained with conventional SE pulse sequences take longer and are prone to motion artifacts; thus, supplementary techniques, such as fat suppression, are manda-tory. 11 Unlike conventional SE technique, fast SE (FSE) or turbo SE (TSE) imaging can be performed in significantly less time, in 1 or 2 breath-holds. 12 Fast or turbo spin-echo sequences are at least equivalent to conventional SE sequences for abdominal lesion detection and characterization. 13-16 There are, however, important differences in image contrast between fast and conventional T2W images. 17 Fat has higher signal intensity, and magnetic susceptibility artifacts of iron and other metals are minimized on "fast" images, compared with conventional SE images. As a consequence, using FSE or TSE techniques is advantageous when imaging patients with metallic stents, surgical clips, or embolization coils. However, magnetization transfer is much greater on "fast" images and, therefore, this effect may lower the signal intensity of solid pancreatic neoplasms. Finally, because the phase-encoding signals from shorter T2 tissues acquired at the end of the echo train have low signal intensity, these tissues may be blurred on "fast" images.
Single-shot fast spin-echo (SSFSE) or half-Fourier acquisition single-shot turbo spin-echo (HASTE) are sophisticated methods of obtaining breath-hold T2W scans by acquiring approximately half of the k-space in one long echo train after a single excitation. 18 The TR of these sequences is infinite. Some studies suggest that such breath-hold sequences can replace conventional T2W images and even "fast or turbo" SE images when appropriately optimized. 19,20 The major advantage of SSFSE or HASTE is that the individual images are acquired in <1 second, allowing motion-free images in patients, even without patient cooperation. Two disadvantages of these sequences, when compared with conventional FSE or TSE, include a lower signal-to-noise ratio (since fewer signals are acquired) and significant blurring of tissues with shorter T2 (ie, solid tissues) because of T2 decay during the long echo train. 20
Normal pancreatic parenchyma has a shorter T2 than most abdominal organs and, therefore, exhibits low-to-intermediate signal on T2W images (Figure 1). 8 The liver and, especially, the spleen have a longer T2 than the pancreas and demonstrate normally higher signal intensity than the pancreas on T2W images.
T1-weighted and T2W images through the pancreas should be acquired axially, although examinations in other planes may be useful in determining the organ of origin of a lesion or in resolving potential problems with partial-volume artifact. Field-of-view should be appropriate to the size of the patient. Slice thickness should not be >8 mm, and there should be no more than a half-slice gap between slices. In general, a slice thickness of 4 to 5 mm is optimal for most applications.
Intravenous administration of extracellular contrast agent
The intravenous (IV) administration of an extracellular contrast agent (gadolinium chelate) is a useful adjunct in the MRI examination of the pancreas. 21 Theoretically, it is desirable to acquire images before and after contrast administration using the same technique. Typically, these are T1W images, which may be obtained with SE or, preferentially, fat-suppressed GRE techniques, as described above. Preferably, postgadolinium imaging is performed in different phases of perfusion (Figure 1).
Administration of gadolinium chelates increases the differences in signal intensity between normal pancreatic parenchyma and the usually less vascular neoplastic tissue. Gadolinium-enhanced MRI is also useful in evaluating acute pancreatitis, especially for the detection of necrosis. 22-24 It can also help differentiate hypervascular pancreatic masses that may simulate cystic lesions on noncontrast scans.
Magnetic resonance pancreatography
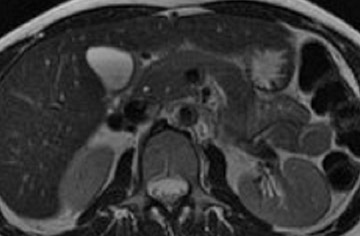
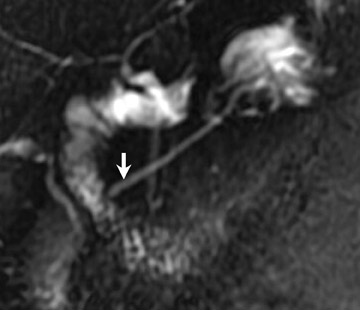
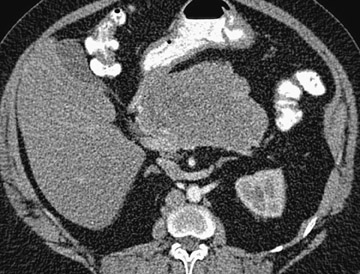
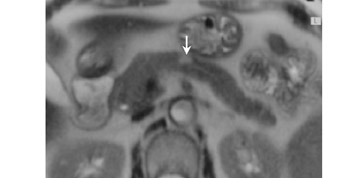
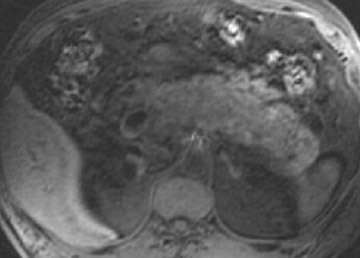
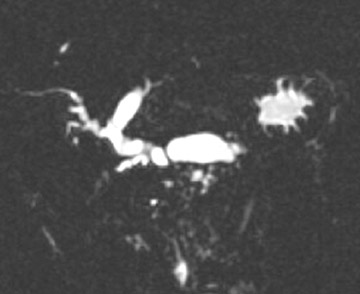
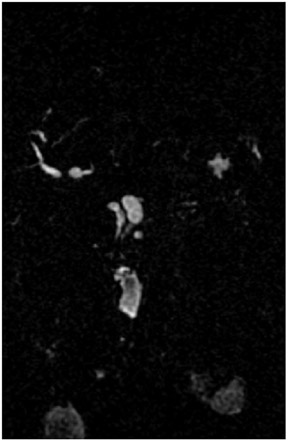
Magnetic resonance pancreatography typically employs "heavily" T2W images of the pancreatic duct, in which pancreatic juices or other stationary or slowly moving fluids appear hyperintense relative to other abdominal tissues. Heavily T2W images are breath-hold "fast" sequences with an extended TE (minimum TE of 160 msec). 19 Two different MRP "snapshot" techniques are usually applied: Thin-section SSFSE or HASTE and thick-section rapid acquisition with relaxation enhancement (RARE) (Figure 2).
MR pancreatography has several advantages over endoscopic retrograde pancreatography (ERP): It is noninvasive, requires no anesthesia, and does not use ionizing radiation. With MRP, there is no increased risk of acute pancreatitis, and it can be performed on patients with altered pancreaticobiliary anatomy following surgery and in patients with complete obstruction of the ducts, in which case it can also demonstrate the upstream anatomy and periductal abnormalities. 25
Magnetic resonance angiography
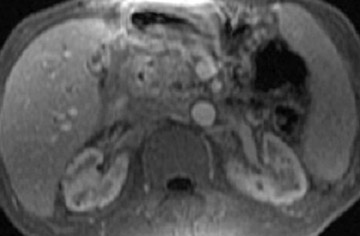
Currently, 3-dimensional gadolinium-enhanced magnetic resonance angiography (3D Gd-MRA) is the best technique to evaluate the peripancreatic vessels by MRI, especially in patients with pancreatic neoplasm, to exclude arterial or venous invasion and to diagnose anatomical vascular abnormalities or variants. 26 The signal obtained by 3D Gd-MRA is determined by the local concentration of gadolinium in the vessels; therefore, acquisition following an optimal time delay is mandatory. This approach also enables one to calculate subtraction images that highlight venous anatomy selectively, to remove disturbing high-intensity background tissue, or to evaluate flow-patterns or organ perfusion.
Pancreatic MRI contrast media
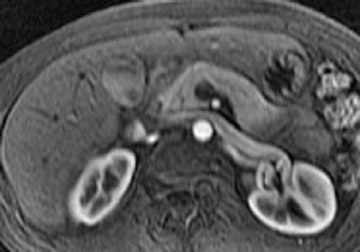
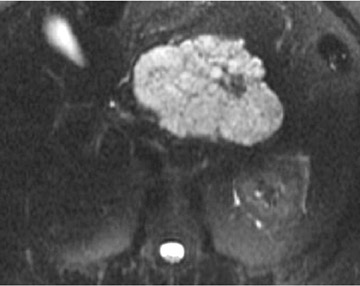
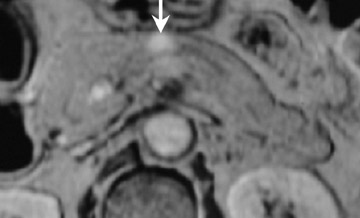
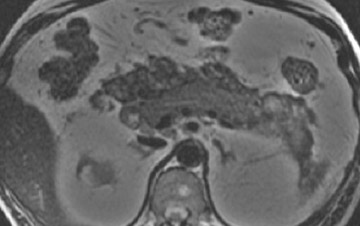
Some specific pancreatic MRI contrast media are currently under investigation, such as manganese dipyridoxyl diphosphate (MnDPDP). A specific pancreatic contrast agent (previously used for liver MRI), MnDPDP causes enhancement of the pancreas on T1W images (a "positive" contrast agent). 27,28 Therefore, unenhanced pancreatic adenocarcinoma can be differentiated from the normal-enhancing pancreas (Figure 3). 29 Although this seems to be especially useful in the diagnosis of smaller tumors and in the differentiation between pancreatic carcinomas and chronic pancreatitis, studies have shown that MnDPDP-enhanced MRI seems to be less sensitive than spiral CT for these purposes. 29,30 Therefore, the question remains whether the use of this agent really improves the detection rate of pancreatic tumors.
Experimentally, target-specific MRI of pancreatic receptors obtained by the administration of monocrystalline iron oxide labeled with cholecystokinin has been evaluated. 31 However, additional studies are necessary to define their precise role in pancreatic MRI.
The MRI protocol currently used at our institution for the evaluation of the pancreas is summarized in Table 1.
Pancreatic MRI: Indications
General indications
The general indications for pancreatic MRI can be divided into 3 main categories: 1) suboptimal or equivocal CT or sonography findings with a high clinical suspicion of pancreatic pathology; 2) contraindications to iodinated contrast administration, including contrast allergy history and renal failure; and 3) contraindications to ionizing radiation (eg, pregnant women or children). 10,32,33
Specific indications
The specific indications for pancreatic MRI include the following: Characterization of suspected parenchymal abnormalities found on CT or ultrasound (US); detection and staging of pan-creatic neoplasms as an adjunct to CT; characterization of cystic pancreatic lesions; detection of small non-organ-deforming pancreatic ductal adenocarcinomas; detection of endocrine tumors; evaluation of acute and chronic pancreatitis when CT is not diagnostic, especially to distinguish chronic pancreatitis from neoplasm; detection of choledocholithiasis as a cause of acute pancreatitis; detection of intraluminal pancreatic duct calculi; and staging of chronic pancreatitis. 7
MRI of pancreatic neoplasms
Pancreatic tumors that originate primarily in the pancreas can be epithelial or nonepithelial, can arise in the exocrine or endocrine pancreas, can appear cystic or solid, or can be secondary (Table 2). 34 The triple role of MRI in evaluating pancreatic neoplasms is tumor detection, characterization, and staging.
Ductal pancreatic adenocarcinoma
Ductal pancreatic adenocarcinoma accounts for approximately 90% of all malignant pancreatic neoplasms. 35 Approximately 80% of the tumors occur in patients 60 to 80 years of age, affecting men about twice as often as women. 36 In up to 70% of cases, the tumor is located within the pancreatic head. 37 Because of the close relationship of the pancreatic head to the common bile duct and duodenum, pancreatic adenocarcinoma in the head generally presents at an earlier stage than do tumors in the pancreatic body or tail. At the time of clinical presentation, two thirds of patients have an advanced tumor stage, with metastatic disease present in 85% of cases. 38 As a distinct capsule does not confine the pancreas, invasion of surrounding structures, especially peripancreatic vessels, is common. 39
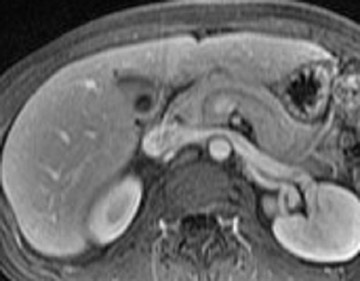
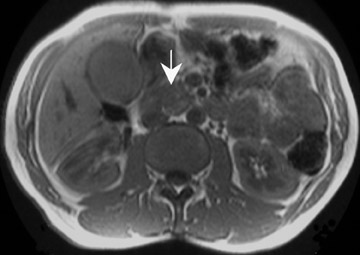
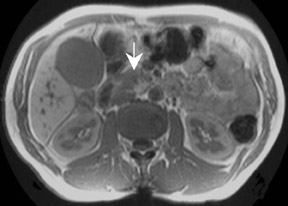
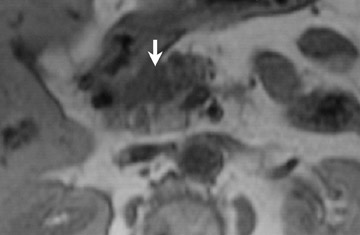
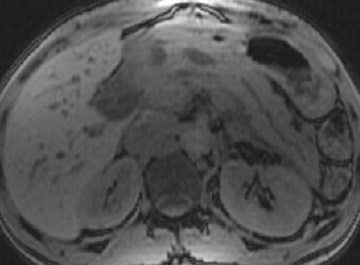
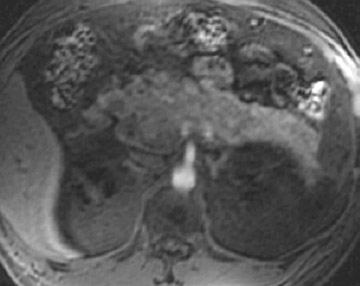
Detection of pancreatic adenocarcinoma is based on unenhanced T1W (fat-suppressed) images and pancreatic-phase postgadolinium T1W spoiled GRE images. 40 On T1W (fat- suppressed) images, pancreatic cancer appears as a low-signal-intensity mass (Figure 4A), and small tumors are clearly separated from normal hyperintense pancreatic tissue. 36 Pancreatic ductal adenocarcinoma is typically a hypovascular lesion (due to abundant desmoplasia) and appears as a low-signal-intensity mass on T1W images during the pancreatic-phase after gadolinium administration (Figure 4). 41-43 On T2W images, the tumor usually presents as an isointense or hyperintense lesion (Figure 4). Secondary findings associated with pancreatic adenocarcinoma are pancreatic duct dilatation (50%), atrophy of the tail of the pancreas (20%), and dilated collateral veins that suggest venous invasion. Postgadolinium breath-hold GRE images are also important for the detection of hepatic metastases, specifically during the portal venous phase.
Cystic epithelial pancreatic neoplasms
Cystic epithelial pancreatic neoplasms, such as serous cystic tumors, mucinous cystic tumors, solid pseudopapillary tumors, and intrapapillary mucinous neoplasms (IPMN) have distinctively different MRI features when compared with pancreatic adenocarcinoma.
Serous cystadenoma- Serous cystadenoma is generally considered to be a benign neoplasm, with only sporadic reports of malignant degeneration. 44 It classically presents as a serous microcystic adenoma and, less commonly, as an oligocystic (macrocystic) adenoma. Serous microcystic adenomas represent 2% of all pancreatic neoplasms, occur most frequently in elderly patients (mean age: 65 years), are more common in women (70%), and have a propensity for occurrence in the pancreatic head. 44 Although most serous cystic tumors are isolated lesions, an association with von Hippel-Lindau disease does exist. 44 On pathology, a serous microcystic adenoma is a large (mean diameter 6 cm) mass that consists predominantly of multiple (>6 cysts), small (<2 cm diameter) cysts that are divided by thin septations. This well-circumscribed tumor is often lobulated and contains a central, stellate, calcified scar. The presence of ≥6 small cysts within the mass is suggestive of serous cystic rather than mucinous cystic neoplasm. 44,45 This tumor is typically not associated with pancreatic duct dilatation or atrophy of the tail of the pancreas.
MRI shows the well-delineated contour of these tumors, the thin septations, and their cystic components. Serous cystic tumors are usually markedly hyperintense on T2W MRI, although some central areas of low signal intensity may occasionally be seen that are caused by the presence of a fibrous scar or calcification (Figure 5). 36 On T1W MR images, the tumor is of low signal intensity, except in cases in which hemorrhage is present. 36 The tumor is hypervascular, secondary to its rich subepithelial capillary network.
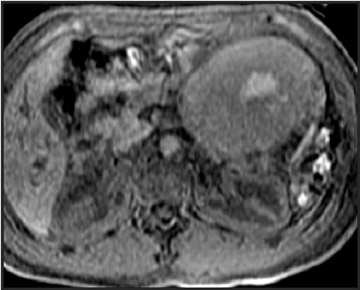
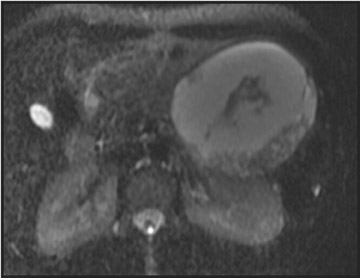
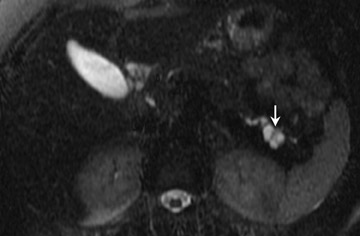
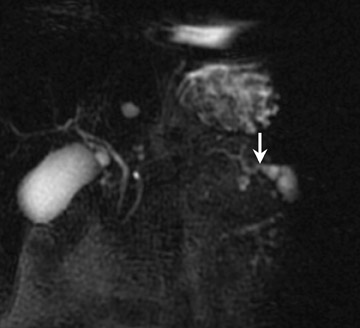
Mucinous cystic tumors- Mucinous cystic tumors range from tumors with malignant potential to frankly malignant cystadenocarcinoma (ie, mucinous cystadenoma, mucinous borderline tumor, and mucinous cystadenocarcinoma). The mucinous macrocystic adenoma is a benign lesion, is more common in women (90%), and usually presents in the 5th decade (mean age 45 years). Approximately 85% of these neoplasms arise in the tail or body of the pancreas. Most typically, these solitary, large (mean diameter 6 to 10 cm), hypovascular tumors are multilocular with <6 individual cysts measuring >2 cm in size. 45 They contain mucin and usually have a thick wall, internal septations, solid papillary excretions, and, occasionally, peripheral calcifications. 37,44
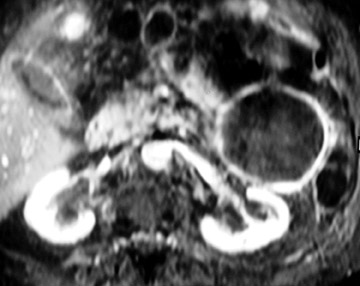
MRI shows the unilocular (Figure 6) or multilocular nature of this mass; scans obtained after IV administration of gadolinium may show enhancement of the septations and the peripheral wall (Figure 6). 45 MR images show the content of these cystic masses to be variable in signal intensity; this variability is related to increased proteinaceous content of the fluid. 45
Solid pseudopapillary tumor- Solid pseudopapillary tumor (also called solid and papillary epithelial neoplasm , papillary cystic tumor , or solid-cystic tumor ) is a benign or low-grade malignancy that represents 1% to 2% of all pancreatic tumors. They usually present in young women (mean age 30 years) and do not show any race or location predilection. 46 This solitary, large (mean diameter 10 cm), well-demarcated mass contains a capsule and is composed of both solid and cystic areas. Internal hemorrhage, characterized by high T1 (Figure 7) and low T2 signal intensity (Figure 7), is a classic MR feature, as is a thick solid capsule that enhances after contrast injection. 46
Intrapapillary mucinous neoplasm- A relatively recent and increasingly reported entity, IPMN is recognized more often now than it was previously probably because of the increasing use of imaging modalities, such as CT and MRP. 47-49 This distinct mucin-producing tumor is thought to originate in the main pancreatic duct or its side branches. When arising from a side branch, they are most commonly located in the uncinate process. They have either papillary hyperplastic, atypical, or malignant epithelium that can cause local and vascular invasion and distant metastases. 47,48 They present at a mean age of 65 years and are slightly more common in men (60%). 50
This tumor typically appears on MRI as a uni- or multilocular cystic lesion (Figure 8) combined with a dilated main pancreatic duct because of marked mucin secretion. 47-48 These tumors can be solitary, multiple, or diffuse sessile. Communication between the main pancreatic duct and the cystic lesion may be depicted, especially with MRP (Figure 8). 47 The most specific predictive signs of a malignant IPMN tumor are the presence of a solid mass, main pancreatic duct dilatation (>10 mm), diffuse or multifocal involvement, and attenuating or calcified intraluminal content. 48
Acinar cell carcinoma and pancreatoblastoma- Other epithelial exocrine pancreatic tumors, such as acinar cell carcinoma and pancreatoblastoma, are very rare; however, pancreatoblastoma is the most common pancreatic tumor in children. Both tumors are more prevalent in males and present as a large mass; acinar cell carcinoma presents at a mean age of 65 and pancreatoblastoma at a mean of 4 years of age. 36
Endocrine pancreatic tumors
Endocrine pancreatic tumors, formally known as islet cell tumors, are benign or malignant neoplasms with endocrine cell differentiation. 51,52 They can be categorized into hyperfunctioning (insulinoma, gastrinoma, glucagonoma, vipoma, and somatostatinoma) and nonhyperfunctioning tumors that can be located in the pancreas or peripancreatic tissue. They are uncommon tumors (1% to 2% of all pancreatic tumors) that usually present in the 6th decade of life; no significant difference in gender distribution has been reported. 53
Generally, endocrine tumors become clinically apparent because of their hormone release and associated symptoms or because of effects related to their size, especially with the nonhyperfunctional tumors. 53 It can be difficult to assess malignancy in islet cell tumors. While direct invasion by the tumor into adjacent organs and metastases are macroscopic signs of malignancy, the only reliable histologic marker is vascular invasion. The likelihood of malignancy increases along with tumor size (93% of tumors >6 cm are malignant), heterogeneity, multiplicity, and presence of calcifications.
Insulinoma- Insulinoma is the most common endocrine tumor of the pancreas. Its clinical onset is typically associated with the Whipple triad (starvation attack, hypoglycemia after a fasting period, and relief by IV dextrose). 51,52 At the time of diagnosis, these tumors are usually (in ~90% of the cases) solitary, small (<2.0 cm), intrapancreatic lesions. Local invasion and distant metastases are present in 25% and 10% of cases, respectively. Malignant insulinomas present with a larger diameter (mean diameter6.2 cm). In only 5% to 10% of cases, multiple insulinomas are found, and there is a slightly higher incidence in the body and tail. 36,53 The classic findings of insulinoma are a solid, homogenous, hypervascular mass, as detailed below.
Gastrinoma- Gastrinoma is the second most common endocrine tumor of the pancreas. The clinical presentation is determined by excessive gastrin production, which induces hypersecretion of gastric acid, followed by peptic ulcerations and diarrhea (Zollinger-Ellison syndrome). This typically small tumor (range 1 to 4 cm) occurs in up to 90% of cases within the "gastrinoma triangle" (superior margin-junction of the cystic and common bile duct; inferior margin-second and third part of the duodenum; medial margin-junction between head and body of the pancreas). 51 Forty percent of gastrinomas are located outside the pancreas, and distant metastases are detected in 25% of cases at the time of diagnosis. Gastrinoma usually presents as a solid homogenous lesion; associated calcification is present in 20% of the masses.
Glucagonomas- Glucagonomas are uncommon endocrine pancreatic tumors that are almost always located in the pancreas and associated with local invasion and distant metastases in 40% and 55% of the cases, respectively. 51 Glucagonoma syndrome includes necrolytic migratory erythema, stomatitis, angular cheilitis, glossitis, diabetes mellitus, anemia, achlorhydria, weight loss, and hypercoagulability. The tumors are found predominantly in the pancreatic body and tail and tend to be larger (mean diameter 6 cm) than insulinomas or gastrinomas. Fifty percent of cases present as solitary, heterogeneous, cystic lesions.
Vipomas- Up to 8% of hyperfunctional pancreatic tumors are vipomas, of which 60% are malignant. 54 These tumors are characterized by the secretion of vasoactive intestinal peptide, which produces the Verner-Morrison syndrome (watery diarrhea, hypokalemia, achlorhydria). These tumors occur mostly in the pancreatic body and tail, with diameters ranging between 4 and 10 cm. 41
Somatostatinoma- Somatostatinoma is the most rare endocrine pancreatic tumor (<1%) and, at the time of diagnosis, most are malignant (75%). 36 The clinical symptoms are related to the complex effects of the suppression of growth hormone, thyroid-stimulating hormone, insulin, glucagon, gastric acid, pepsin, and secretin. As a result, the patients present with diabetes mellitus, gallstones, low gastric output, and weight loss.
Nonhyperfunctioning endocrine tumors- As a group, nonhyperfunctioning endocrine tumors are the third most common islet cell neoplasms, accounting for about 15% of all endocrine pancreatic tumors. 52 They usually cause no symptoms until patients present with stomach or biliary tract obstruction. At that time, 90% of the tumors are malignant, and distant metastases and local invasion are present in 25% and 50% of the cases, respectively. Because of their large size (diameter >10 cm in 30% of cases), the tumors tend to necrose and hemorrhage.
The challenge that cross-sectional imaging faces today is to identify small hyperfunctioning tumors and to stage large hyperfunctioning and nonhyperfunctioning tumors for malignancy. 51,52 Since the majority of hyperfunctioning islet cell tumors are very small, frequently measuring <3 cm in size, this can be a difficult task. Islet cell tumors present as hypervascular lesions, best seen in the early phases of pancreatic enhance- 51,52 The most striking evidence of malignancy is evidence of metastatic disease to the liver or local lymph nodes. In contrast to ductal adenocarcinoma, vascular encasement is usually not seen macroscopically.
On MRI, insulinomas and gastrinomas appear as lesions with low signal intensity on T1WI (Figure 9) and high signal intensity on T2WI (Figure 9). 36 Fat-suppression sequences are useful in emphasizing the signal intensity differences between tumor and normal hyperintense pancreatic tissue. 36 The use of IV gadolinium-DTPA is helpful, particularly in the detection of islet cell tumors, since they are hypervascular (Figure 51,54 Ringlike enhancement in the periphery of the tumor is frequently seen, while the center may remain hypointense secondary to fibrosis. The more uncommon but generally larger hyperfunctioning (eg, glucagonoma, vipoma, somatostatinoma) and nonhyperfunctioning tumors present with a slightly lower signal intensity on T1WI and a moderate signal intensity on T2WI. 36,51,54 Contrast administration will reveal an enhancement pattern similar to that of the smaller insulinomas or gastrinomas. In large tumors, hemorrhage or necrosis may occur and each is easily depicted. 54
Nonepithelial tumors- Nonepithelial tumors such as lymphoma, teratoma, lymphangioma, lipoma, and neural tumors can occur in the pancreas. Lymphoma can cause multiple or solitary lesions that are associated with adenopathy and advanced systemic involvement (Figure 10).
Metastatic disease- Metastatic disease can also spread to the pancreas, most often from primary renal, lung, or breast cancer, as well as melanoma. Metastases typically occur in the presence of advanced metastatic disease.
MRI in pancreatitis
The role of radiologic imaging in patients with suspected pancreatitis is to help confirm the diagnosis, try to establish the cause of the disease, assess the severity, and detect complications.
Acute pancreatitis
Acute pancreatitis is an acute inflammatory process of the pancreas, with variable involvement of other regional tissues or remote organs systems. 55 The 3 main causes include cholelithiasis (38%), alcoholism (35%), and idiopathic etiologies. 56 On pathology, pancreatitis can be classified as mild (edematous gland and/or spotty peripancreatic fatty tissue necrosis) or severe (alternating chalky white fat necrosis and hemorrhage and/or pancreatic necrosis). CT is the modality of choice in acute pancreatitis evaluation due to its accuracy, reproducibility, and availability. 57
In the assessment of acute pancreatitis, MRI can evenly depict the presence and extent of necrosis and peripancreatic fluid collections and is superior to CT in the detection of mild acute pancreatitis. 58 A routine pancreas protocol including T2WI, fat-suppressed T1WI, and a series of T1WI GRE sequences prior to and immediately following gadolinium administration is a reliable means of staging acute pancreatitis and reaching a prognosis. 59 In severe acute pancreatitis, gadolinium-enhanced MRI is particularly useful for the assessment of pancreatic parenchymal perfusion and the presence of necrosis. The enlargement of the gland is well visualized on any sequence, and parenchymal edema is better shown on unenhanced T1WI (Figure 11). T2-weighted sequences are the most sensitive in revealing fluid collections. Furthermore, by using additional MRP or MRA, MRI allows the accurate diagnosis of underlying etiologies, such as choledocholithiasis or pancreas divisum, and vascular complications.
Chronic pancreatitis
Chronic pancreatitis is an irreversible clinical disorder represented on pathology by irregular fibrosis and cellular infiltration, ductal abnormalities, and loss of exocrine and endocrine pancreatic function. 60 MRI examination should include T1W and T2W images and MRP. Chronic pancreatitis presents with decreased T1 and variable T2 signal intensity. The enhancement after gadolinium administration is reduced and delayed. With MRP, the extent of morphologic ductal changes as well as the severity of the disease can be assessed and classified into 4 categories: Equivocal (<3 abnormal side branches); mild (at least 3 abnormal side branches); moderate (main pancreatic duct changes); and marked (main pancreatic duct with severe dilatation of >1 cm, marked mural irregularity, obstruction, or intraductal filling defects) (Figure 12). 61
Several centers have investigated functional MR evaluation of the pancreas by obtaining MRP images before and after IV secretin administration. After slow IV administration, secretin will stimulate the secretion of fluid and bicarbonate by the exocrine pancreas and increase the tonus of the sphincter of Oddi, thus improving the visualization of the pancreatic duct and its side branches. 62 Furthermore, the volume of effluent into the duodenal lumen can be graded, allowing a relative estimation of the exocrine reserve. 62 Currently, the main indications for functional imaging are the characterization of filling defects, the assessment of exocrine function in the presence of parenchyma atrophy, and a better evaluation of the papillary region.
An area of difficulty for all imaging modalities has been differentiating focal pancreatitis from pancreatic adenocarcinoma. Both types of lesions occur most often in the pancreatic head, may cause bile duct obstruction, and may present in the absence of calcification and duct strictures or dilatation. 60 However, patients with an inflammatory mass are likely to be younger at the time of presentation and have a history of alcoholism and previous episodes of acute or chronic pancreatitis. 25 Several studies have suggested that MRI can help differentiate these 2 pathologies, especially with respect to focal or diffuse changes in signal intensity and contrast enhancement. On fat-suppressed T1WI, areas of chronic pancreatitis show diminished signal intensity compared with normal pancreas but the degree of signal reduction is usually less than that associated with carcinoma. 60 Inflammatory masses in chronic pancreatitis may result in signal intensities comparable with those of the liver, whereas carcinoma often shows signal intensities hypointense to the liver. 10 The "duct penetrating sign" on MRP images-a smoothly stenotic or normal main pancreatic duct penetrating into a mass-has been reported to occur more frequently in inflammatory pancreatic masses than in pancreatic carcinoma. 60 Further study, however, showed that MRI could not reliably differentiate the 2 conditions. 63 The use of MnDPDP may offer the potential to distinguish pancreatic carcinoma from chronic focal pancreatitis, but further studies are necessary.
Autoimmune pancreatitis is a special form of chronic pancreatitis that is unique in its effective clinical response to steroid therapy. 60 On MRI, it presents as a diffuse or focal pancreatic enlargement associated with narrowing of the main pancreatic duct and decreased and increased signal intensity on T1W and T2W sequences, respectively. 60 In addition, contrast-enhanced imaging may show a peripancreatic rim of decreased enhancement surrounding the pancreas and the common bile duct on the arterial phase images. 60
Conclusion
MRI plays a triple role in the evaluation of the pancreas: Diagnosis, staging, and detection of complications. The role of MRI has increased, especially in imaging patients with suspected pancreatic neoplasms. Currently, major MRI indications include assessment of neoplasms (especially cystic pancreatic tumors) and evaluation of chronic pancreatitis.
REFERENCES
- Gauger J, Holzknecht NG, Lackerbauer CA, et al. Breathhold imaging of the upper abdomen using a circular polarized-array coil: Comparison with standard body coil imaging. MAGMA. 1996;4:93-104.
- Soyer P, Bluemke DA, Rymer R. MR imaging of the liver: Technique. Magn Reson Imaging Clin N Am. 1997;5:205-221.
- Martin J, Sentis M, Puig J, et al. Comparison of in-phase and opposed-phase GRE and conventional SE MR pulse sequences in T1-weighted imaging of liver lesions. J Comput Assist Tomogr. 1996;20:890-897 .
- Yamashita Y, Yamamoto H, Tomohiro N, et al. Phased array breath-hold versus non-breath-hold MR imaging of focal liver lesions: A prospective comparative study. J Magn Reson Imaging. 1997;7:292-297.
- Haase A, Frahm J, Matthaei D, et al. FLASH imaging, rapid NMR imaging using low flip-angle pulses. J Magn Reson Imaging. 1986;67:258-266.
- Semelka RC, Marcos HB. Nonendocrine tumors of the pancreas. In: Heuck E, Reiser M, eds. Magnetic Resonance Imaging of the Abdomen. Berlin: Springer-Verlag; 1998:117-124.
- Ly JN, Miller FH. MR imaging of the pancreas: A practical approach. Radiol Clin North Am. 2002; 40:1289-1306.
- Winston CB, Mitchell DG, Outwater EK, Ehrlich SM. Pancreatic signal intensity on T1-weighted fat saturation MR images: Clinical correlation. J Magn Reson Imaging. 1995;5:267-271.
- Semelka RC, Kroeker MA, Shoenut JP, et al. Pancreatic disease: Prospective comparison of CT, ERCP, and 1.5-T MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology. 1991;181:785-791.
- Semelka RC, Ascher SM. MR Imaging of the pancreas. Radiology. 1993;188:593-602.
- Felmlee JP, Ehman RL. Spatial presaturation: A method for suppressing flow artifacts and improving depiction of vascular anatomy in MR imaging. Radiology.1987;164:559-564.
- Hennig J, Nauerth A, Friedburg H. RARE imaging: A fast imaging method for clinical MRI. Magn Reson Med.1986;3:823-833.
- Carpenter KD, Macaulay SE, Schulte SJ, et al. MR of focal liver lesions: Comparison of breath-hold and non-breath-hold hybrid RARE and conventional spin-echo T2-weighted pulse sequences. J Magn Reson Imaging. 1996;6:596-602.
- Catasca JV, Mirowitz SA. T2-weighted MR imaging of the abdomen: Fast spin-echo vs conventional spin-echo sequences. AJR Am J Roentgenol. 1994; 162:61-67.
- Low RN, Francis IR, Sigeti JS, Foo TK. Abdominal MR imaging: Comparison of T2-weighted fast and conventional spin-echo, and contrast-enhanced fast multiplanar spoiled gradient-recalled imaging. Radiology. 1993;186:803-811.
- Outwater EK, Mitchell DG, Vinitski S. Abdominal MR imaging: Evaluation of a fast spin-echo sequence. Radiology. 1994;190:425-429.
- Constable RT, Anderson AW, Zhong J, Gore JC. Factors influencing contrast in fast spin-echo MR imaging. Magn Reson Imaging. 1992;10:497-511.
- Kiefer B, Grassner J, Hausman R. Image acquisition in a second with half Fourier acquisition single-shot turbo spin-echo. J Magn Reson Imaging. 1994;4 (P suppl):86-87.
- Bosmans H, Van Hoe L, Gryspeerdt S, et al. Single-shot T2-weighted MR imaging of the upper abdomen: Preliminary experience with double-echo HASTE technique. AJR Am J Roentgenol. 1997;169: 1291-1293.
- Semelka RC, Kelekis NL, Thomasson D, et al. HASTE MR imaging: Description of technique and preliminary results in the abdomen. J Magn Reson Imaging. 1996;6:698-699.
- Gabata T, Matsui O, Kadoya M, et al. Small pancreatic adenocarcinomas: Efficacy of MR imaging with fat suppression and gadolinium enhancement. Radiology. 1994;193:683-688.
- Piironen A, Kivisaari R, Pitkaranta P, et al. Contrast-enhanced magnetic resonance imaging for the detection of acute haemorrhagic necrotizing pancreatitis. Eur Radiol. 1997;7:17-20.
- Chezmar JL, Nelson RC, Small WC, Bernardino ME. Magnetic resonance imaging of the pancreas with gadolinium-DTPA. Gastrointest Radiol. 1991;16: 139-142.
- Freeny PC. Radiology of the pancreas. Curr Opin Radiol.1991;3:440-452.
- Robinson PJ, Sheridan MB. Pancreatitis: Computed tomography and magnetic resonance imaging. Eur Radiol. 2000;10:401-408.
- Prince MR, Yukel EK, Kaufman JA, et al. Dynamic gadolinium-enhanced three-dimensional abdominal MR arteriography. J Magn Reson Imaging.1993;3: 877-881.
- Gehl HB, Urhahn R, Bohndorf K, et al. Mn-DPDPin MR imaging of pancreatic adenocarcinoma: Initial clinical experience. Radiology. 1993; 186:795-798.
- Kettritz U, Warshauer DM, Brown ED, et al. Enhancement of the normal pancreas: Comparison of manganese-DPDP and gadolinium chelate. Eur Radiol.1996;6:14-18.
- Mayo-Smith WW, Schima W, Saini S, et al. Pancreatic enhancement and pulse sequence analysis using low-dose mangafodipir trisodium. AJR Am J Roentgenol. 1998;170:649-652.
- Rieber A, Tomczak R, Nussle K, et al. MRI with mangafodipir trisodium in the detection of pancreatic tumours: Comparison with helical CT. Br J Radiol. 2000;73:1165-1169.
- Reimer P, Weissleder R, Shen T, et al. Pancreatic receptors: Initial feasibility studies with a targeted contrast agent for MR imaging. Radiology.1994;193:527-531.
- Mammone JF, Siegelman ES, Outwater EK. Magnetic resonance imaging of the pancreas and biliary tree. Semin Ultrasound CT MR. 1998;19:35-52.
- Paley MR, Ros PR. MR imaging of the liver-a practical approach. Magn Reson Imaging Clin N Am. 1997;5:415-429.
- Solcia E, Capella C, Klöppel G. AFIPAtlas of T, Series III, Tumor Pathologyumors of the Pancreas.1997.
- Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455-465.
- Mergo PJ, Helmberger TK, Buetow PC, et al. Pancreatic neoplasms: MR imaging and pathologic correlation. RadioGraphics. 1997;17:281-301.
- Friedman AC. Pancreatic neoplasm. In: Gore RM, Levine MS, Laufer I, eds. Textbook of Gastrointestinal Radiology. Vol 2. Philadelphia, PA: WB. Saunders Co; 1994;2161-2186.
- Steele GD Jr, Osteen RT, Winchester DP, et al. Clinical highlights from the National Cancer Data Base: 1994. CACancer J Clin. 1994;44:71-80.
- Chen J, Baithun SI. Morphological study of 391 cases of exocrine pancreatic tumours with special reference to the classification of exocrine pancreatic carcinoma. J Pathol. 1985;146:17-29.
- Semelka RC, Kroeker MA, Shoenut JP, et al. Pancreatic disease: Prospective comparison of CT, ERCP, and 1.5-T MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology. 1991;181:785-791.
- Ichikawa T, Haradome H, Hachiya J, et al. Pancreatic ductal adenocarcinoma: Preoperative assessment with helical CT versus dynamic MR imaging. Radiology.1997;202:655-662.
- Nishiharu T, Yamashita Y, Abe Y, et al. Local extension of pancreatic carcinoma: Assessment with thin-section helical CT versus with breath-hold fast MR imaging-ROC analysis. Radiology. 1999;212: 445-452.
- Kanematsu M, Shiratori Y, Hoshi H, et al. Pancreas and peripancreatic vessels: Effect of imaging delay on gadolinium enhancement at dynamic gradient-recalled-echo MR imaging. Radiology. 2000;215: 95-102.
- Ros PR, Hamrick-Turner JE, Chiechi MV, et al. Cystic masses of the pancreas. RadioGraphics. 1992;12:673-686.
- Scott J, Martin I, Redhead D, et al. Mucinous cystic neoplasms of the pancreas: Imaging features and diagnostic difficulties. Clin Radiol. 2000;55:187-192.
- Cantisani V, Mortele KJ, Levy A, et al. MR imaging features of solid pseudopapillary tumor of the pancreas in adult and pediatric patients. AJR Am J Roentgenol.2003;181:395-401.
- Sugiyama M, Atomi Y, Hachiya J. Intraductal papillary tumors of the pancreas: Evaluation with magnetic resonance cholangiopancreatography. Am J Gastroenterol. 1998;93:156-159.
- Taouli B, Vilgrain V, Vullierme MP, et al. Intraductal papillary mucinous tumors of the pancreas: Helical CT with histopathologic correlation. Radiology. 2000;217:757-764.
- Taouli B, Vilgrain V, O'Toole D, et al. Intraductal papillary mucinous tumors of the pancreas: Features with multimodality imaging. J Comput Assist Tomogr. 2002;26:223-231.
- Suzuki Y, Atomi Y, Sugiyama M, et al. Cystic neoplasm of the pancreas: A Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004;28:241-246.
- Chung MJ, Choi BI, Han JK, et al. Functioning islet cell tumor of the pancreas. Localization with dynamic spiral CT. Acta Radiol.1997;38:135-138.
- Procacci C, Carbognin G, Accordini S, et al. Nonfunctioning endocrine tumors of the pancreas: Possibilities of spiral CT characterization. Eur Radiol.2001;11:1175-1183.
- Rossi P, Allison DJ, Bezzi M, et al. Endocrine tumors of the pancreas. Radiol Clin North Am. 1989;27:129-161. Erratum in: Radiol Clin North Am.1989;27:following xii.
- Mortele KJ, Oei A, Bauters W, et al. Dynamic gadolinium-enhanced MR imaging of pancreatic VIPoma in a Patient with Verner-Morrison syndrome. Eur Radiol.2001;11:1952-1955.
- Bradley EL3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg. 1993;128:586-590.
- Mortele KJ, Banks PA, Silverman SG. State-of-the-art imaging of acute pancreatitis. JBR-BTR.2003;86:193-208.
- Piironen A. Severe acute pancreatitis: Contrast-enhanced CT and MRI features. Abdom Imaging. 2001;26:225-233.
- Amano Y, Oishi T, Takahashi M, Kumazaki T. Nonenhanced magnetic resonance imaging of mild acute pancreatitis. Abdom Imaging. 2001;26: 59-63.
- Lecesne R, Taourel P, Bret PM, et al. Acute pancreatitis: Interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology. 1999;211:727-735.
- De Backer AI, Mortele KJ, Ros RR, et al. Chronic pancreatitis: Diagnostic role of computed tomography and magnetic resonance imaging. JBR-BTR. 2002;85:304-310.
- Axon AT. Endoscopic retrograde cholangiopancreatography in chronic pancreatitis. Cambridge classification. Radiol Clin North Am. 1989;27: 39-50.
- Matos C, Metens T, Deviere J, et al. Pancreatic duct: Morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology.1997; 203: 435441.
- Johnson PT, Outwater EK. Pancreatic carcinoma versus chronic pancreatitis: Dynamic MR imaging. Radiology. 1999;212:213-218.