Imaging the female pelvis: When should MRI be considered?
Images
Dr. Hubert is a Radiology Resident and Dr. Bergin is an Associate Professor of Radiology, Thomas Jefferson University Hospital, Philadelphia, PA.
Ultrasound is the imaging modality of choice for the female pelvis. It is widely available, has broad acceptance by patients as a “familiar test,” and is relatively inexpensive. High-resolution imaging of transvaginal ultrasound provides high diagnostic accuracy for pelvic pathology. However, there are some shortcomings with this modality, such as the limited field of view, obscuration of pelvic organs by the presence of bowel gas, inherent limitations dependent on patient size, and its dependence on the skill and experience of the operator. The American College of Radiology has provided guidelines for when ultrasound is an appropriate imaging tool (Table 1) for the evaluation of the female pelvis.1
With its high contrast resolution, its ability to provide good tissue characterization, and its multiplanar imaging capabilities, magnetic resonance imaging (MRI) is increasingly used to evaluate pelvic pathology (Table 2).2-5 There is a significant difference, however, in the inherent costs of MRI and ultrasound. The dilemma for referring physicians and general radiologists is to decide when it is appropriate to refer patients for MRI.5 This article describes in what situations MRI should be considered to evaluate the female pelvis.
Technique
Standard MRI of the female pelvis at our institution includes coronal single-shot fast spin-echo (FSE), axial T2-weighted (T2W) FSE, axial in-phase and opposed-phase T1-weighted (T1W) gradient-recalled echo (GRE), and sagittal T2W FSE fat-suppressed sequences utilizing a dedicated pelvic phased-array coil (see Table 3).
Fat-suppressed axial 3-dimensional T1W GRE dynamic imaging following intravenous administration of 20 mL of gadolinium contrast is obtained routinely. Delayed fat-suppressed 2-dimensional (2D) GRE imaging is subsequently obtained in another plane. If artifact from the bowel is perceived as problematic on initial sequences, glucagon may be administered by intramuscular (0.8 mg) or intravenous injection (0.2 mg). This is not routinely used in the author’s institution, however. For pelvic floor imaging, dynamic 2D GRE imaging may be performed with and without the Valsalva maneuver, as discussed later, to detect pelvic prolapse.
Adnexal masses
In the premenopausal woman, the ovaries are typically well evaluated by ultrasound. When evaluating an adnexal mass on ultrasound, the diagnostic challenges that may arise include accurately localizing the mass, determining whether or not it is ovarian in origin, and, when complex, whether it is definitively benign or malignant. Many adnexal masses are benign and, when indicated, can be treated surgically by laparoscopic technique. Complex echogenic adnexal lesions on ultrasound may represent hemorrhagic cysts, endometrioma, dermoids, or ovarian neoplasms. If a cystic adnexal mass >5 cm in a premenopausal woman or >3 cm in a postmenopausal woman persists or increases in size on follow-up ultrasound, MRI should be considered so that malignancy can be excluded.5-8 MRI should also be considered when a solid or solid cystic adnexal lesion with internal color flow is detected by ultrasound. MRI has an overall accuracy of 91% to 93% in the characterization of adnexal masses as benign or malignant. In these circumstances, it has been found that the use of MRI is cost-effective in that it reduces unnecessary surgical procedures.
Ovarian cyst
A simple unilocular ovarian cyst is not an indication for MRI, as it is a common incidental finding in both pre- and postmenopausal women. These cysts are well evaluated by ultrasound. However, the postmenopausal ovary tends to contain fewer cysts of smaller size. When they are <3 cm in size with a wall ≤3 mm and have the characteristic appearance of low T1 and high T2 signal intensity, these cysts can be considered benign in both populations. Studies that specifically examined the premenopausal ovary have shown that the risk of malignancy for unilocular cysts <5 cm in an asymptomatic woman approaches zero.
Hemorrhagic ovarian cyst and endometrioma
With its high contrast resolution and its tissue characterization capabilities, MRI is a valuable tool for the characterization of echogenic adnexal masses detected by ultrasound. The accuracy of MRI for identifying lesions such as hemorrhagic cysts and endometriomas is higher than with transvaginal ultrasound imaging. On ultrasound, these lesions are occasionally misinterpreted as solid tumors, mature cystic teratomas, or complex cysts.5-8
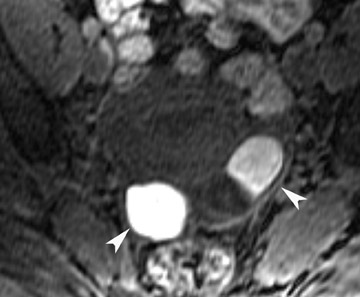
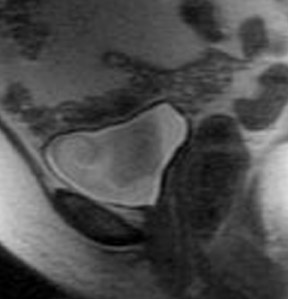
Most ovarian cysts are functional in origin and occasionally can be complicated by intracystic hemorrhage. The MRI characteristics can be variable in this situation, depending on the age and amount of the hemorrhagic component. In general, however, they tend to be of relatively high signal intensity on T1W images and of intermediate-to-high signal intensity on T2W images and frequently reveal a fluid-fluid level (Figure 1). Hemorrhagic cysts should remain of relatively high signal on T1W images with fat suppression, which helps to differentiate them from dermoid cysts in most situations. They also tend to have thicker walls than do simple cysts and may exhibit wall enhancement on postcontrast images.
However, the internal components of the cysts should never enhance. The majority of hemorrhagic ovarian cysts may be accurately diagnosed by ultrasound. MRI, however, should be considered when the hemorrhagic cystic lesion persists or increases in size on follow-up ultrasound.
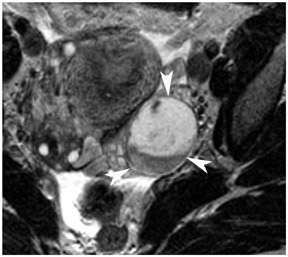
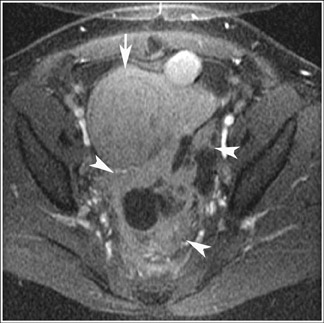
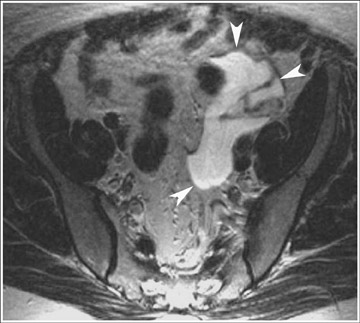
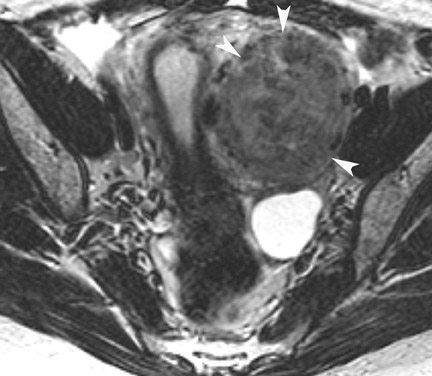
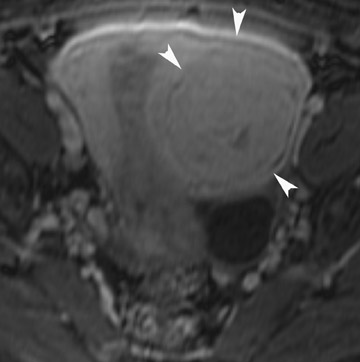
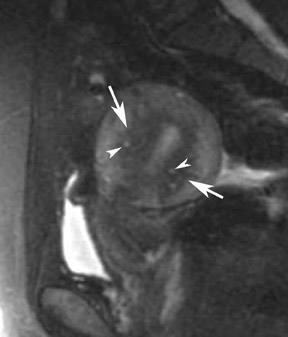
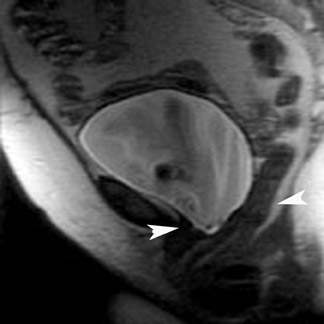
Endometriosis is the ectopic presence of functional endometrial glands and stroma outside the uterus. Although laparoscopy remains the standard for diagnosing and staging pelvic endometriosis, MRI can identify lesions obscured at laparoscopy by dense ad hesions. MRI has a sensitivity of 90%, a specificity of 98%, and an overall accuracy of 96% for the identification of endometriomas in patients with clinically suspected adnexal masses.8-10 As with hemorrhagic cysts, the MRI appearance of endometriomas is somewhat variable based on the stage and amount of blood products. They are typically of high signal on T1W images and of intermediate-to-low signal intensity on T2W images (Figure 2). This relatively lower signal intensity on T2W imaging, which is often referred to as “T2-shading,” is secondary to methemoglobin, protein, and iron from repeated episodes of hemorrhage. Endometriomas are more frequently bilateral and usually exhibit multiplicity. Endometriosis implants on serosal or peritoneal surfaces are identified on MRI by high T1 signal seen on nonenhanced fat-suppressed images.
In diagnosing ovarian torsion, a true gynecologic emergency ultrasound is the modality of choice. If sonographic results are equivocal, however, MRI may be performed. Ovarian torsion is usually the consequence of an underlying ovarian lesion, most commonly dermoid or parovarian cysts.9 As with hemorrhagic cysts and endometriomas, the presence of hemorrhage leads to a variable appearance that can be further complicated by the presence of infarction. Findings on MRI that suggest ovarian torsion include deviation of the uterus toward the affected side, engorgement of blood vessels toward the affected side, and a small amount of ascites. Findings that indicate a twisted adnexal tumor include protrusion of the lesion to the affected side, thick straight blood vessels draping over the lesion, and complete absence of enhancement.
Fallopian tube abnormalities
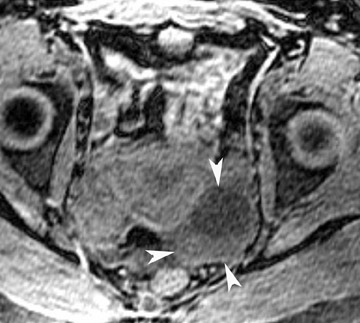
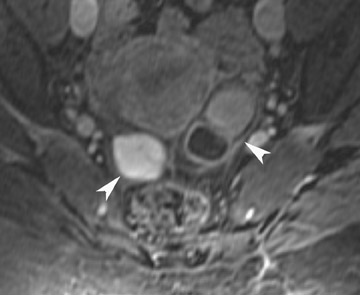
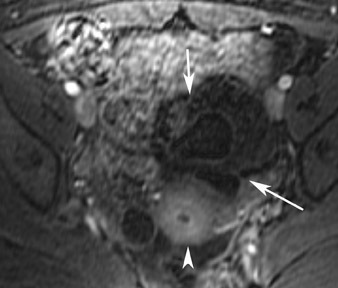
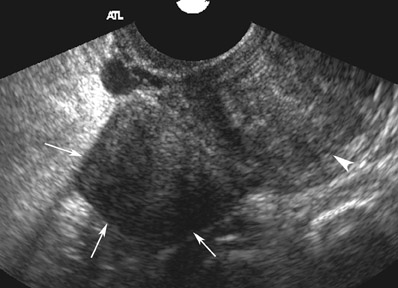
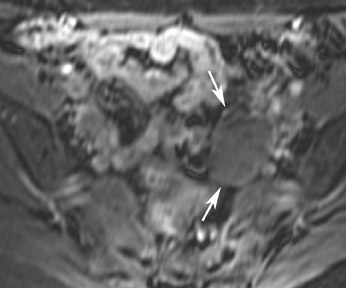
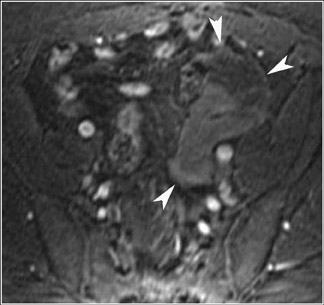
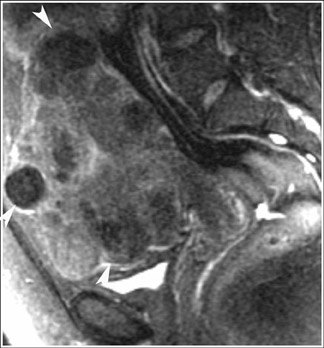
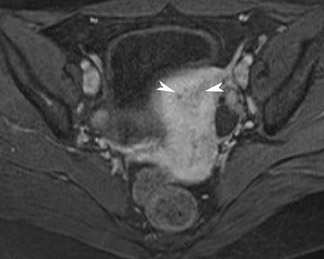
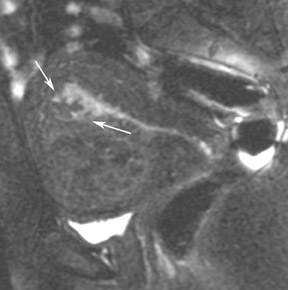
Abnormalities of the fallopian tube commonly present as adnexal masses and generally result from salpingitis, endometriosis, or peritubal adhesions.8 Although readily identified with ultrasound, MRI is useful in cases of dense scarring and in the identification of the adjacent ovary. With a large simple hydrosalpinx, the relatively large field of view utilized in MRI (as compared with ultrasound) facilitates easy recognition of its tubular morphology, which differentiates it from a potential ovarian cystic mass. When hydrosalpinx is complicated by pyosalpinx, the T2 signal intensity may exhibit shading or hypointense areas from the high protein content, similar to that of an endometrioma. When complicated by a tubo-ovarian abscess, inflammatory changes are seen in the surrounding tissue. The associated inflammatory changes and the abscess wall exhibit intense enhancement with contrast administration (Figure 3).
Dermoid
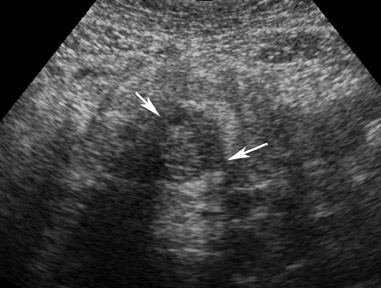
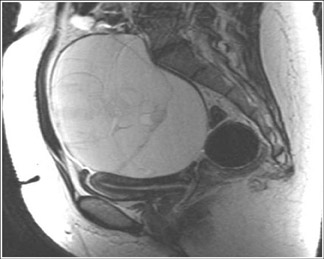
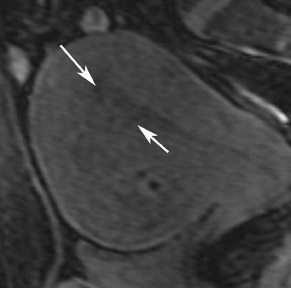
Germ cell tumors represent 15% to 20% of all tumors of the ovary. Dermoids account for 95% of all ovarian germ cell tumors. Most of these are unilocular, contain sebaceous fluid, and are commonly referred to as mature cystic teratomas or dermoid cysts. Although these are usually asymptomatic and are incidental findings in young women, the standard treatment is surgical removal because of their potential to cause ovarian torsion or for the cyst to rupture. There is also a rare chance of malignant degeneration to squamous cell carcinoma. Although most mature cystic teratomas can be diagnosed at ultrasound, one prospective study has shown the sensitivity to be 58% with a specificity of 99%.11 Numerous pitfalls exist in their diagnosis by ultrasound. The presence of blood clot within a hemorrhagic cyst can appear echogenic, which causes confusion in the diagnosis. Adjacent echogenic bowel can also be mistaken for a mature cystic teratoma and vice versa.
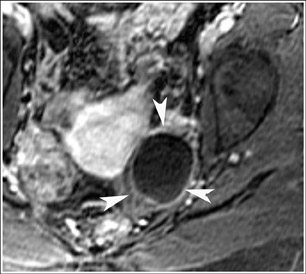
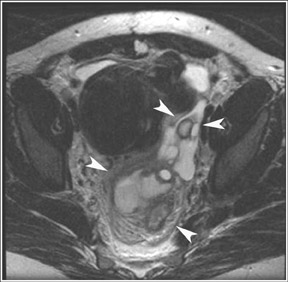
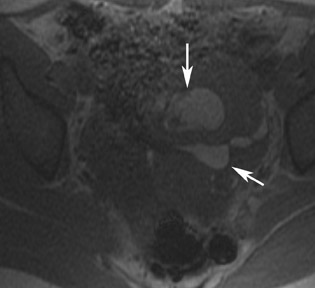
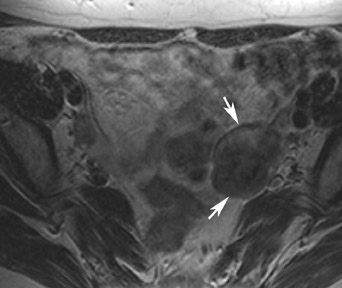
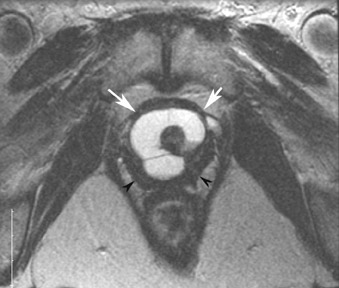
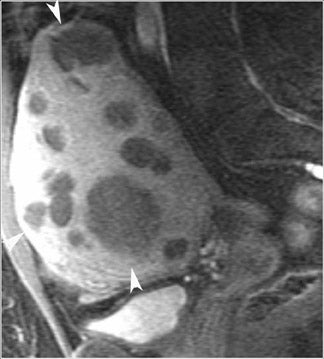
MRI has a high sensitivity for the presence of fat within the sebaceous component, which is characteristic of nearly all these lesions. The sebaceous component is of very high signal intensity on T1W images and is somewhat variable on T2W images.10-12 Fat suppression can differentiate macroscopic fat from other hemorrhagic lesions that appear hyperintense on T1W images, such as hemorrhagic cysts and endometriomas (Figure 4). Even the rare lesion that contains microscopic fat can be differentiated by using chemical shift imaging with the use of in- and out-of-phase sequences. Mature cystic teratomas also commonly have a solid mural nodule that is referred to as a dermoid plug or a Rokitansky nodule. Although rare, malignant transformation can occur in 1% to 2% of cases. In these cases, the women tend to be postmenopausal and the images are characterized by transmural extension of the solid component and, often, by direct invasion of adjacent pelvic structures.
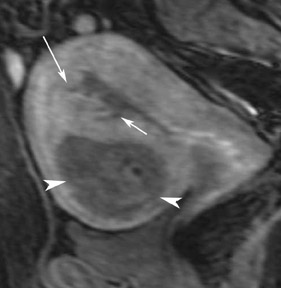
Fibrous tumors
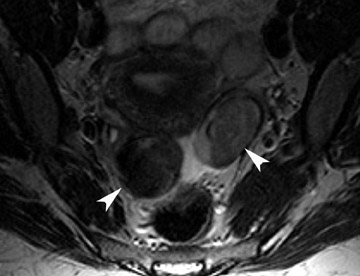
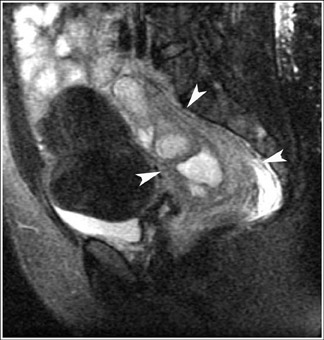
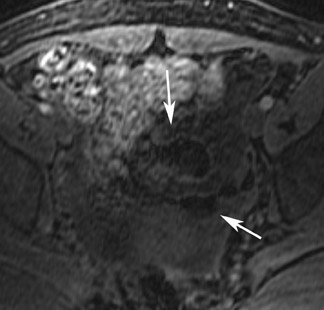
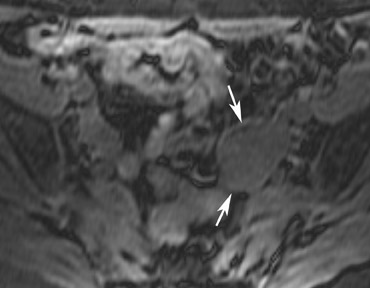
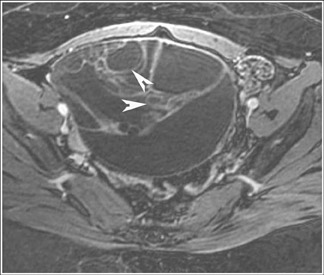
The most common subtypes of sexcord stromal tumors are fibromas, fibrothecomas, thecomas, and Brenner tumors. Although these account for only 4% of all ovarian tumors, they are the most common solid primary tumor of the ovary. These benign lesions are composed of dense fibrous tissue. On ultrasound, they appear as solid hypoechoic masses exhibiting marked attenuation. It is often difficult to distinguish fibromas and fibrothecomas from other solid ovarian masses and pedunculated fibroids on ultrasound. On MRI, they exhibit low signal intensity on T2W images with low-to-intermediate signal on T1W images (Figure 5).13 Small areas of cystic degeneration and edema may be present in larger lesions, and they tend to show mild enhancement with gadolinium contrast. Although similar in MRI appearance to uterine leiomyomas, their ovarian origin can be confirmed by the presence of follicles in the surrounding ovarian tissue. MRI can also exclude these lesions by identifying the ovaries as separate from the lesion.13
Ovarian malignancies
Ovarian carcinoma remains the leading cause of gynecological cancer-related deaths in the United States, and it is estimated to comprise 6% of all cancer-related deaths in 2006.14 Approximately 75% of women have advanced disease at the time of diagnosis with a 5-year survival of only 29% in those with metastases.12-14
No effective screening method currently exists for the detection of ovarian cancer, in part because the preclinical phase is estimated to be <2 years. Current methods include transvaginal ultrasound and determining serum CA-125 levels. Unfortunately, CA-125 is not specific for ovarian cancers. Although MRI is clearly not cost-effective as a screening tool, it has become quite valuable for patients in whom sonographic results are indeterminate. As previously discussed, MRI can accurately characterize benign masses such as teratomas and endometriomas; this has proven to be a cost-effective approach, since unnecessary surgery can be avoided.10-12 MRI is also superior to CT in the diagnosis of peritoneal implants and has superior accuracy in diagnosing ovarian malignancy compared with CT and Doppler sonography. Gadolinium-enhanced MRI has a rate of depiction of benign lesions of 93% and of malignant lesions of 95%.10-12
Primary ovarian neoplasms are classified as epithelial tumors (60% to 70%), germ cell tumors (15% to 20%), and sexcord stromal tumors (5% to 10%). However, no imaging modality can differentiate between neoplastic subtypes. A study by Hricak et al3 found that the 2 most significant predictors of malignancy were the presence of vegetations in a cystic lesion and the presence of necrosis in a solid lesion.14 Other predictors of malignancy include wall or septal thickness >3 mm, presence of ascites, and a maximum diameter greater than 4 to 6 cm (Figure14-17 On T2W images, an ovarian mass of high signal intensity that is found in conjunction with implants in the abdomen and pelvis is suggestive of mucinous cystadenocarcinoma with peritoneal metastatic disease. Because of the inherent wide field of view of MRI relative to pelvic ultrasound, a single MRI examination can not only characterize an ovarian mass but can also be used in staging when a mass is noted to have malignant features. The presence of ascites, peritoneal, or serosal metastases as well as hydronephrosis may be detected.
Nonovarian adnexal masses
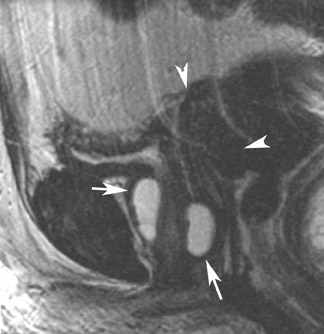
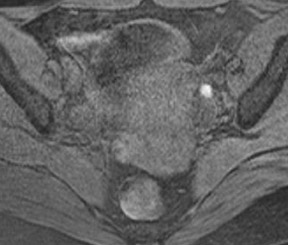
Its relatively large field of view also allows MRI to localize pelvic lesions and their origin more accurately. Identification of a pelvic mass separate from the uterus and ovary changes the differential diagnosis. Examples of such lesions that may be accurately diagnosed by MRI include peritoneal inclusion cysts (Figure 7), pedunculated fibroid cysts, para-ovarian cysts, paratubal cysts, or lymphadenopathy. Cystic midline lesions that are accurately characterized by MRI include Gartner’s duct cyst, Bartholin’s cyst, and urethral diverticula (Figure 8).
Uterine anatomy
Uterine anatomy is well delineated by MRI. The 3 distinct zones (which are most clearly delineated on sagittal T2W images) are the outer myometrium (intermediate-to-high signal), the inner myometrium or junctional zone (low signal), and the endometrial complex (high fluid/near fluid signal). Endometrial thickness varies greatly, depending on the phase of the menstrual cycle and the age of the patient. It is generally<15 mm in premenopausal women and <8 mm in postmenopausal women, regardless of cycle or hormonal stimulation. The junctional zone appears as low signal intensity on T2W images and typically measures <12 mm. The intrinsic T2 contrast between the outer myometrium and the junctional zone becomes less marked in the postmenopausal uterus because of reduced fluid in the tissues.
Leiomyomas
Leiomyomas, benign uterine neoplasms, are the most common tumor of the female genital tract. Their classification is based on their location within the uterine corpus as either intramural, submucosal, or subserosal. Most women are asymptomatic; however, the most common symptom is bleeding. Transvaginal ultrasound has been shown to be as efficient as MRI in the detection of the presence of myomas; however, MRI is superior in terms of mapping individual myomas.18 This is especially true with larger uteri and with the presence of a large number of myomas.
On MRI, a uterus containing leiomyomas will be enlarged and will have an abnormal contour. On T2W images, leiomyomas appear as sharply marginated lesions of low signal intensity relative to the myometrium (Figure 9). Often, a high-signal-intensity rim can be identified, more commonly in intramural or subserosal leiomyomas. Leiomyomas may contain calcifications, especially in older women. Calcified myomas can cause significant artifact on ultrasound and can obscure adjacent tissues. While similar calcification appears as a signal void on MRI, it typically does not limit the evaluation of adjacent tissues. On MRI, myomas larger than 3 to 5 cm are often heterogeneous because of various degrees of degeneration. Although varied, enhancement tends to be heterogeneous and less than that of the myometrium.19-21
MRI is the modality of choice in evaluating leiomyomas before and after treatment with uterine artery embolization (UAE).20,21 The use of MRI is optimal for pre-embolization assessment for delineating the location of leiomyoma and for accurately assessing pedunculated lesions. It is particularly useful in providing postembolization comparative images to assess whether there are persistent enhancing fibroids and to compare pre- and posttherapeutic size. Pre-embolization MRI may also be used to predict collateral feeding vessels by modifying protocol to optimize angiographic imaging. MRI can also identify or exclude the presence of other uterine abnormalities that may impact or preclude treatment.22 Various studies have shown that certain preprocedural imaging characteristics may accurately predict response to UAE. High signal intensity on T1W sequences may indicate pre-existing hemorrhagic infarction, resulting in poor outcome secondary to insufficient volume reduction. The degree of contrast enhancement has been shown to correlate with tumor response. A complete lack of contrast enhancement indicates nonviable tumor that will not respond to treatment.22,23 After successful UAE, there is an overall reduction in uterine size and in mean leiomyoma volume. MRI characteristics that indicate a successful treatment include high signal intensity on T1W images and homogenously decreased T2 signal intensity. These findings are suggestive of hemorrhagic infarction and correlate with a lack of contrast enhancement (Figure 10).22-24 This lack of contrast enhancement has been shown to persist as far out as 3 years postembolization. Similarly, a lack of infarction at short-term follow-up will likely persist at long-term follow-up with MRI.
Adenomyosis
Adenomyosis is the presence of ectopic endometrial glands from the basal layer of the endometrium within the myometrium, often associated with myometrial hyperplasia. It is a common gynecologic disorder that most commonly affects premenopausal women. Symptoms are nonspecific and include pelvic pain, dysmenorrhea, menorrhagia, and abnormal uterine bleeding.25 Al-though adenomyosis is typically a diffuse process, focal areas of involvement are also seen. It is these focal lesions that are often mistaken for leiomyomas. It is important to differentiate between them, as their treatments vary greatly. Although gonadotropin-releasing hormone analog therapy or endometrial ablation can be performed, the definitive treatment for severe cases of adenomyosis is ultimately hysterectomy. With the advent of more conservative therapies such as embolization therapy, MRI may play a role in monitoring treatment response.26
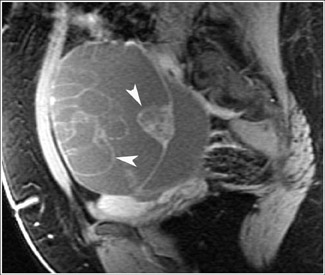
Studies have shown that MRI is superior to ultrasound for the diagnosis of adenomyosis.26 The characteristic appearance of adenomyosis on MRI is diffuse thickening (>12 mm) of the junctional zone. This is most evident on T2W sequences and corresponds to the smooth muscle hyperplasia associated with the ectopic tissue. A junctional zone ≤8 mm virtually excludes the disease, whereas a width of 9 to 11 mm is equivocal. Other findings that may suggest the diagnosis include poorly defined margins of the junctional zone and foci of high signal intensity on T1W or T2W sequences that indicate the presence of endometrial cysts (Figure 11). The foci of high signal may represent ectopic endometrium, cystically dilated endometrial glands, or hemorrhage.27-29 Contrast administration has been shown to be of very little benefit in the diagnosis of adenomyosis.
Distinguishing between focal adenomyosis and leiomyomas is reliably achieved with MRI, and we now know that these conditions often coexist.29 Unlike leiomyomas, a focal area of adenomyosis will have indistinct margins and extend subjacent to the myometrium. The signal characteristics will otherwise be the same as with the diffuse form of the disease. Treatment implications for focal adenomyosis and leiomyoma differ, however, so accurate diagnosis is important.
Uterine endometrial pathology
Endometrial thickening seen at ultrasound is a nonspecific finding.30 For years, endometrial biopsy was the diagnostic standard for differentiating benign causes, such as polyps and leiomyomas, from endometrial carcinoma. However, benign abnormalities far outnumber cancer in these situations.30 Other problems with biopsy include vaginal or cervical stenosis and difficulty in obtaining an adequate specimen. MRI can be helpful in further differentiating these lesions.
Endometrial polyps
Endometrial polyps typically present with postmenopausal bleeding, particularly in patients on tamoxifen therapy. Transvaginal ultrasound has high sensitivity and specificity for endometrial polyps.31,32 Sonohysterography is the most sensitive and specific imaging modality.
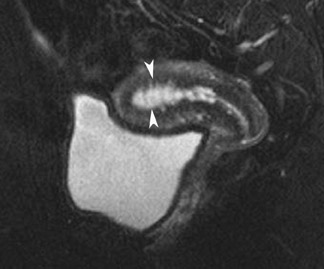
On MRI, endometrial polyps are of intermediate signal intensity on T1W images and of intermediate-to-high signal intensity on T2W images. Their signal intensity on T2W images tends to be higher than that seen in endometrial carcinoma. The presence of a central focus of low signal intensity on T2W images indicates a fibrous core, which suggests the diagnosis of an endometrial polyp (Figure 12).33 They are also more likely than endometrial carcinoma to contain intramural cysts,32-34 which appear as smooth-walled, well-defined cystic structures of high signal intensity on T2W images. However, these cysts may also be observed in endometrial carcinoma. Utilizing contrast enhancement significantly improves lesion detection. However, enhancement patterns do not reliably distinguish endometrial carcinoma from other lesions. Although these imaging features can help distinguish polyps from endometrial carcinoma, it is often not specific enough to avoid the need for biopsy. Moreover, these 2 conditions frequently coexist.34,35
Endometrial carcinoma
Endometrial carcinoma is the fourth most common cancer in women.36-39 The disease occurs most commonly in women in the sixth and seventh decades of life. Clinically, patients present with abnormal uterine bleeding in 75% to 90% of cases. As a result of the early clinical symptoms, patients often present with early-stage disease.34
Adenocarcinoma represents roughly 90% of endometrial carcinomas, with varying grades of differentiation. Other histologic subtypes include squamous, papillary, and clear-cell carcinoma. However, histology cannot be determined based on imaging characteristics.38,39
MRI is not recommended as a screening procedure in the diagnosis of endometrial carcinoma. However, MRI has proven to be an important tool for the staging of known endometrial carcinoma.38 MRI can differentiate between superficial and deep-muscle–invasive tumors by using a combination of T2W imaging and contrast-enhanced MRI. This can significantly alter surgical management. The presence of cervical invasion also alters preoperative and surgical management. MRI has been shown to be superior to both CT and ultrasound in assessing myometrial invasion, cervical extension, and nodal involvement.
Endometrial carcinomas appear isointense to the myometrium and endometrium on T1W images. On T2W images, their signal intensity is commonly hyperintense; however, this is quite variable.38 Endometrial carcinomas usually enhance less than the myometrium does, with the difference less marked on delayed images. Myometrial invasion is best visualized on T2W images, where it appears as a disruption or an irregularity of the junctional zone by a mass of intermediate signal intensity (Figure 13).
Transmyometrial extension of tumor is identified by interruption of the normal low signal intensity of the serosal surface. However, early serosal invasion may be difficult to detect. Parametrial involvement is best depicted on T1W images with a signal intensity change in the parametrial fat. T1-weighted images are also better for identifying tumor involvement of the vagina when there is disruption of the low signal intensity wall. Lymph node involvement is suggested on T1W images with nodes that have a diameter >1 cm in the short axis. MRI can also detect tumor extension outside the true pelvis as well as bladder and rectal invasion.
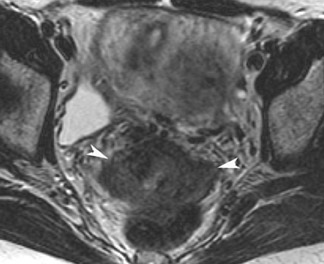
Cervical carcinoma
Like the uterus, the cervix has a zonal anatomy that is well delineated on T2W images. Cervical cancer is the third most common gynecologic malignancy in women. MRI is not initially used to diagnose cervical cancer but is used to stage disease in women who have had a diagnosis established by a Pap smear or biopsy. T2-weighted images obtained in the sagittal plane and in a plane along the short axis of the cervix are the most useful for local staging. On T2W images, cervical cancer appears as a mass of higher signal intensity than the adjacent fibrous cervical stroma, but the mass is of lower signal intensity than the endometrium.33 If the low signal intensity of the inner cervical stroma is preserved, stage IIB or higher disease is excluded, which indicates that the patient is likely a surgical candidate. Macroscopic extension of tumor into the parametrial fat establishes a diagnosis of stage IIB disease. MRI has an accuracy range of 75% to 95% in detecting parametrial invasion (Figure 14). MRI can accurately assess for more advanced disease such as pelvic sidewall invasion and obstruction of the distal ureter.33 Localizing the tumor and determining the presence or absence of ureteral obstruction can provide a road map for radiation therapy.
Dynamic MRI of the pelvic floor
Pelvic floor dysfunction annually affects nearly 300,000 to 400,000 women severely enough to necessitate surgery. Dysfunction of the pelvic floor may manifest as prolapse, incontinence, pelvic pain, or constipation.40
The pelvic floor can be divided into 3 compartments: the anterior compartment, which contains the bladder and urethra; the middle compartment, which contains the vagina, cervix, and uterus; and the posterior compartment, which contains the rectum. All 3 compartments are supported by a complex network of muscles and fascia that form the urogenital diaphragm, or the pelvic floor. Damage to ≥1 of these myofascial elements can lead to individual or multiple organ prolapse, and overall laxity and stretching or tearing can lead to generalized pelvic floor relaxation.
Diagnosis and grading of pelvic floor dysfunction has previously been performed with physical examination and radiographic imaging, such as voiding cystourethrography, evacuation proctography, cystocolpoproctography, and peritoneography. However, the development of fast MRI sequences has allowed for the quick evaluation of pelvic organ prolapse and pelvic floor relaxation with increased patient comfort, decreased complexity, and decreased invasiveness and radiation exposure.41 The intrinsic soft tissue contrast capability of MRI allows for detailed visualization of the pelvic floor, and the faster techniques now allow for dynamic evaluation of pelvic support structures. Studies have shown that dynamic MRI has greater sensitivity than physical examination and has led to changes in the initial surgical plan in 41% of patients. It has become clear that MRI has an important role in the preoperative planning in patients with pelvic floor dysfunction.42,43
Currently, no universally accepted radiologic criteria for assessing pelvic floor dysfunction exist; however, the most widely accepted criteria involve organ movement below the pubococcygeal line (PCL), which extends from the inferior margin of the symphysis pubis to midway between the first and second coccygeal segments. A line that extends from the lowermost aspect of the symphysis pubis to the puborectalis muscle forms the puborectalis hiatus. In the normal patient, the puborectalis hiatus is <6 cm and does not descend >2 cm below the PCL. The upper urethra, urethrovesical junction, bladder, upper vagina, uterus, small bowel, sigmoid colon, mesenteric fat, and rectum should all be above the hiatus. Staging of any prolapse is done in 2-cm increments below the puborectalis hiatus. Small is 0 to 2 cm below, moderate is 2 to 4 cm, and a large prolapse extends >4 cm below this line.
For optimal MRI evaluation in the sagittal plane, the patient is placed in the supine position. Static images are first obtained, with subsequent series of images performed during resting and straining in the midsagittal plane. Selected midline sagittal T2W images at rest and on the Valsalva maneuver and/or sagittal 2D GRE images in real-time at rest and on Valsalva are used to assess the degree of pelvic floor descent and pelvic organ prolapse (Figure 15).42,43 Some authors advocate the use of intraluminal contrast, but this is not used routinely at our institution.
Müllerian duct anomalies
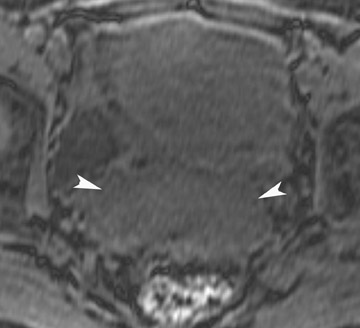
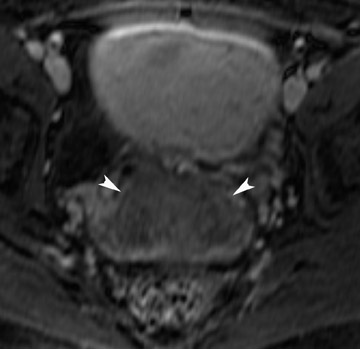
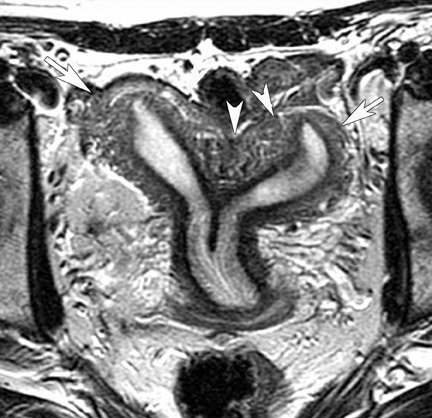
The incidence of müllerian duct anomalies is approximately 0.1% to 3%. Although they are often asymptomatic, obstetrical complications occur in up to 25% of these women, including spontaneous abortion, stillbirth, preterm delivery, and adverse obstetrical outcomes. Knowledge of the type and severity of the anomaly can significantly impact treatment, as the therapies vary greatly. With an accuracy approaching 100%, MRI has become the gold standard in identifying müllerian duct anomalies.44-47 Various studies have shown that it is superior to sonography and hysterosalpingography.46 In patients with primary amenorrhea, MRI can determine the presence or absence of the vagina, cervix, and uterus.47 Bicornuate and septate uteri are the 2 most common types of müllerian ductal anomalies. Differentiating between these 2 entities is important because of their complications and different treatments. The evaluation of the external fundal contour is the key to differentiating between bicornuate and septate uteri (Figure 16). This can be best evaluated on a plane that passes through the long axis of the uterus. The outer contour of the septated uterus is convex or flat, with <10-mm concavity. The outer fundal contour of a bicornuate uterus or uterus didelphys should have >10-mm concavity between the right and left uterine horns.
Conclusion
Ultrasound remains the first line of imaging for the female pelvis, with high diagnostic accuracy rates for uterine and ovarian abnormalities. MRI should be considered for the evaluation of adnexal pathology when sonographic characteristics are not definitive to determine whether an adnexal mass is ovarian in origin and to determine the likelihood of malignancy. MRI has an established role in the pre- and post-procedural assessment for uterine artery embolization, diagnosis of adenomyosis, staging of known endometrial and cervical carcinoma, evaluation of suspected müllerian ductal anomalies, and presurgical workup for pelvic floor prolapse. Other indications not discussed here include assessment of the pregnant patient with acute pelvic pain and of fetal anatomy. In most cases, fetal anatomy is well evaluated by ultrasound, but MRI can play a role in problem solving. For the acute pelvis and when there is a concern for acute appendicitis, the role of MRI has yet to be established. Beyond the period of organogenesis, CT may be considered. Within the period of organogenesis, however, MRI is a safe alternative, and limited studies to date have shown promising results.
REFERENCES
- ACR Practice Guideline for the Performance of Pelvic Ultrasound in Females. Reston, VA: American College of Radiology;2006.
- ACR Practice Guideline for the Performance of Magnetic Resonance Imaging (MRI) of the Soft Tissue Components of the Pelvis. Reston, VA: American College of Radiology;Amended 2006.
- Hricak H, Chen M, Coakley F, et al. Complex adnexal masses: Detection and characterization with MR imaging–multivariate analysis. Radiology. 2000;214:39-46.
- Sohaib SA, Sahdev A, Van Trappen P, et al. Characterization of adnexal mass lesions on MR imaging. AJR Am J Roentgenol. 2003;180:1297-1304.
- Schwartz LB, Panageas E, Lange R, et al. Female pelvis: Impact of MR imaging on treatment decisions and net cost analysis. Radiology. 1994;192:55-60.
- Outwater EK, Mitchell DG. Normal ovaries and functional cysts: MR appearance. Radiology. 1996;198:397-402.
- Bailey CL, Ueland FR, Land GL, et al. The malignant potential of cystic ovarian tumors in women over 50 years of age. Gynecol Oncol. 1998;69:3-7.
- Togashi K, Nishimura K, Kimura I, et al. Endometrial cysts: Diagnosis with MR imaging.Radiology.1991;180:73-78.
- Kimura I, Togashi K, Itoh K, et al. Ovarian torsion: CT and MR imaging appearances. Radiology. 1994; 190:337-341.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: Tumor types and imaging characteristics. RadioGraphics. 2001;21:475-490.
- Mais V, Guerriero S, Ajossa S, et al. Transvaginal ultrasonography in the diagnosis of cystic teratoma. Obstet Gynecol. 1995;85:48-52.
- Kido A, Togashi K, Konishi I, et al. Dermoid cysts of the ovary with malignant transformation: MR appearance. AJR Am J Roentgenol. 1999;172:445-449.
- Troiano RN, Lazzarini KM, Scoutt LM, et al. Fibroma and fibrothecoma of the ovary: MR imaging findings. Radiology. 1997;204:795-798.
- Böhm-Vélez M, Mendelson E, Bree R, et al. Ovarian cancer screening. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215(suppl):861-871.
- Low RN. Magnetic resonance imaging of the abdomen: Applications in the oncology patient. Oncology (Williston Park). 2000;14(6 suppl 3):5-14.
- Kurtz AB, Tsimikas JV, Tempany CM, et al. Diagnosis and staging of ovarian cancer: Comparative values of Doppler and conventional US, CT, and MR imaging correlated with surgery and histopathologic analysis–Report of the Radiology Diagnostic Oncology Group. Radiology. 1999; 212:19-27.
- Funt SA, Hricak H. Ovarian malignancies. Top Magn Reson Imaging. 2003;14:329-337.
- Dueholm M, Lundorf E, Hansen ES, et al. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol. 2002;186:409-415.
- Mittl RL Jr, Yeh IT, Kressel HY. High-signal-intensity rim surrounding uterine leiomyomas on MR images: Pathologic correlation. Radiology. 1991;180:81-83.
- Burn PR, McCall JM, Chinn RJ, et al. Uterine fibroleiomyoma: MR imaging appearances before and after embolization of uterine arteries. Radiology. 2000;214:729-734.
- Harman M, Zeteroglu S, Arslan H, et al. Predictive value of magnetic resonance imaging signal and contrast-enhancement characteristics on post-embolization volume reduction of uterine fibroids. Acta Radiol. 2006;47:427-435.
- Jha RC, Ascher SM, Imaoka I, Spies JB. Symptomatic fibroleiomyomata: MR imaging of the uterus before and after uterine arterial embolization. Radiology. 2000;217:228-235.
- Nikolaidis P, Siddiqi AJ, Carr JC, et al. Incidence of nonviable leiomyomas on contrast material-enhanced pelvic MR imaging in patients referred for uterine artery embolization. J Vasc Interv Radiol. 2005;16:1465-1471.
- Pelage JP, Guaou NG, Jha RC, et al. Uterine fibroid tumors: Long-term MR imaging outcome after embolization. Radiology. 2004;230:803-809.
- Kuligowska E, Deeds L 3rd, Lu K 3rd. Pelvic pain: Overlooked and underdiagnosed gynecologic conditions. RadioGraphics. 2005;25:3-20.
- Ascher SM, Arnold LL, Patt RH, et al. Adenomyosis: Prospective comparison of MR imaging and transvaginal sonography. Radiology. 1994; 190:803-806.
- Reinhold C, McCarthy S, Bret PM, et al. Diffuse adenomyosis: Comparison of endovaginal US and MR imaging with histopathologic correlation. Radiology. 1996;199:151-158.
- Outwater EK, Siegelman ES, Van Deerlin V. Adenomyosis: Current concepts and imaging considerations. AJR Am J Roentgenol. 1998;170:437-441.
- Togashi K, Ozasa H, Konishi I, et al. Enlarged uterus: Differentiation between adenomyosis and leiomyoma with MR imaging. Radiology. 1989; 171:531-534.
- Sheth S, Hamper UM, Kurman RJ. Thickened endometrium in the postmenopausal woman: Sonographic-pathologic correlation Radiology. 1993;187:135-139.
- Ascher SM, Imaoka I, Lage JM. Tamoxifen-induced uterine abnormalities: The role of imaging. Radiology. 2000;214:29-38.
- Grasel RP, Outwater EK, Siegelman ES, et al. Endometrial polyps: MR imaging features and distinction from endometrial carcinoma. Radiology. 2000;214:47-52.
- Hricak H, Finck S, Honda G, Göranson H. MR imaging in the evaluation of benign uterine masses: Value of gadopentetate dimeglumine-enhanced T1-weighted images. AJR Am J Roentgenol.1992;158:1043-1050.
- Manfredi R, Gui B, Maresca G, et al. Endometrial cancer: Magnetic resonance imaging. Abdom Imaging. 2005;30:626-636.
- Larson DM, Connor GP, Broste SK, et al. Prognostic significance of gross myometrial invasion with endometrial cancer.Obstet Gynecol.1996;88:394-398.
- Saez F, Urresola A, Larena JA, et al. Endometrial carcinoma: Assessment of myometrial invasion with plain and gadolinium-enhanced MR imaging. J Magn Reson Imaging. 2000;12:460-466.
- Kim SH, Kim HD, Song YS, et al. Detection of deep myometrial invasion in endometrial carcinoma: Comparison of transvaginal ultrasound, CT, and MRI. J Comput Assist Tomogr. 1995;19: 766-772.
- Yamashita Y, Mizutani H, Torashima M, et al. Assessment of myometrial invasion by endometrial carcinoma: Transvaginal sonography vs contrast-enhanced MR imaging. AJR Am J Roentgenol.1993;161:595-599.
- Chaudhry S, Reinhold C, Guermazi A, et al. Benign and malignant diseases of the endometrium. Top Magn Reson Imaging. 2003;14:339-357.
- DeLancey JO. The hidden epidemic of pelvic floor dysfunction: Achievable goals for improved prevention and treatment. Am J Obstet Gynecol. 2005; 192:1488-1495.
- Kaufman HS, Buller JL, Thompson JR, et al. Dynamic pelvic magnetic resonance imaging and cystocolpoproctography alter surgical management of pelvic floor disorders. Dis Colon Rectum. 2001; 44:1575-1583; discussion 1583-1584.
- Goodrich MA, Webb MJ, King BF, et al. Magnetic resonance imaging of pelvic floor relaxation: Dynamic analysis and evaluation of patients before and after surgical repair. Obstet Gynecol. 1993;82: 883-891.
- Barbaric ZL, Marumoto AK, Raz S. Magnetic resonance imaging of the perineum and pelvic floor. Top Magn Reson Imaging. 2001;12:83-92.
- Siewert B, Hochman M, Levine D. Problems and pitfalls in MR evaluation of uterine anomalies. J Womens Imaging. 2002;4:100-107.
- Troiano RN. Magnetic resonance imaging of mullerian duct anomalies of the uterus. Top Magn Reson Imaging. 2003;14:269-279.
- Pellerito JS, McCarthy SM, Doyle MB, et al. Diagnosis of uterine anomalies: Relative accuracy of MR imaging, endovaginal sonography, and hysterosalpingography. Radiology. 1992;183:795-800.
- Carrington BM, Hricak H, Nuruddin RN, et al. Mullerian duct anomalies: MR imaging evaluation. Radiology. 1990;176:715-720.
Related Articles
Citation
Imaging the female pelvis: When should MRI be considered?. Appl Radiol.
October 11, 2011