Imaging of crystal deposition disease
Images





Dr. Farpour is a research fellow of MSK Radiology, and Dr. Tehranzadeh is Emeritus Professor and Vice Chair of Radiology at UCI, and Chief of Radiology and Nuclear Medicine, VA Long Beach Health Care System-Radiology, Long Beach, CA; and Dr. Maasumi, is a third year post-graduate (PGY3) in the Department of Neurology, Texas Tech University Health Sciences Center, Lubbock, TX.
Magnetic resonance imaging (MRI) is an important diagnostic tool for the evaluation of crystal deposition diseases affecting soft tissues, tendons, ligaments, and joints.1 A number of crystals are associated with joint diseases. There are various imaging features for common crystal-related deposition diseases including monosodium urate, calcium pyrophosphate dihydrate as well as hydroxyapatite depositional diseases.
There are several known causes of crystal deposition diseases involving the soft tissues, bones and joints, specifically gout, calcium pyrophosphate dehydrate, and hydroxyapatite depositional diseases. MRI features associated with crystal deposition diseases include size, soft-tissue signal characteristics, arthritic changes, as well as etiology and common locations of such deposition diseases.
Gout
Gout has a prevalence of about 20 per 1000 patients and an annual incidence of 1 to 2 per 1000 patients. Both the incidence and prevalence of gout have doubled in the United States (U.S.) over the last 50 years, most likely due to an aging population. Gout is the most common inflammatory arthritis in men over the age of 30. Women are infrequently affected.2
The etiology of gout is manifested directly in the deposition of monosodium urate monohydrate crystals or uric acid, due to hyperurecimia defined as >6 mg/dL in women and 7 mg/dL in men. This occurs due to ingestion of purine in our food since uric acid is the nonmetabolized byproduct of purine.2
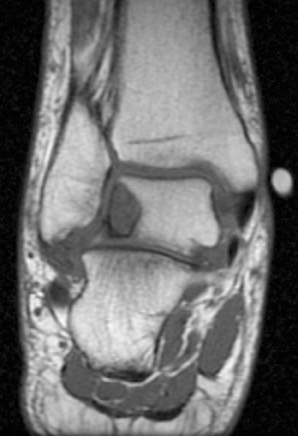
Gout presents as arthropathy, tenosynovitis, bursitis, and cellulitis of noninfectious origin, urinary tract calculi, and very commonly as soft-tissue deposits known as tophi. Chronic gout is often associated with the development of tophaceous deposits within cartilage, synovial membranes, bursae, and tendons. On the other hand, acute gout commonly presents as monoarticular arthritis, occurring in the first metatarsophalangeal (MTP) joint, elbow, and fingers. Although rare, the presence of tophaceous gout at the site of tendon disruption, dorsum of the foot, heel, and ankle (Figure 1) has been reported.3 Gout is a progressive disease, starting as asymptomatic hyperurecimia, acute gouty arthropathy, periods without symptoms, and finally chronic tophaceous gout.4
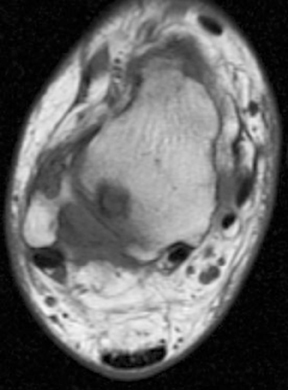
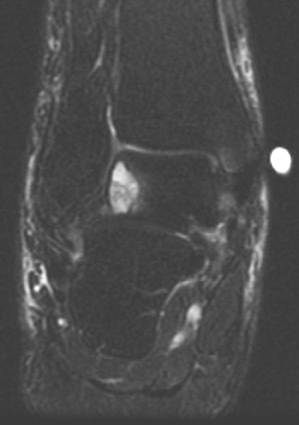
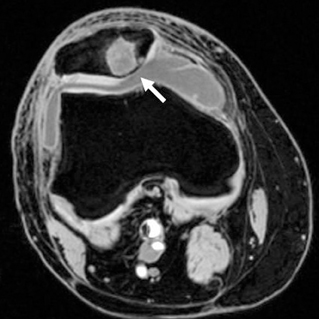
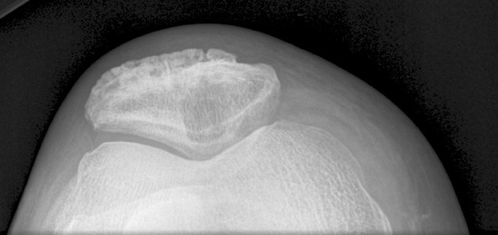
The radiographic findings of gout manifest after 5 to 6 years. The radiographic characteristics of gouty arthritis include sharply defined erosions with hooked margins and calcified, asymmetric, periarticular tophi, so-called erosions with “overhanging edges.” Gout is a monoarticular or oligoarticular disease found often in the appendicular skeleton in order of decreasing frequency: Feet, hands and wrist, elbows, and knees. Gout may present with cystic changes of carpal and tarsal bones. Gout has a tendency for involvement of extensor surfaces. Therefore quadriceps and patellar tendon involvement is common (Figure 2). But it is especially found in the first metatarsophalangeal joint. The tophi are deposits, occurring within the intra-articular synovium and cartilage. Tophaceous deposits account for the bone erosions seen in gouty arthritis. These large erosions with an “overhanging edge” may be para-articular, intra-articular, or located at a considerable distance from the joint. Gouty tophi may produce bony erosions and cystic changes of the adjacent bone resulting in chronically deforming arthritis. Large deposits of urate crystals are also seen in adjacent bursae, tendons, ligaments, and subcutaneous layers of the skin. It can cause limitations of joint movement by involving the joint structure directly or by tendons serving the joint. Gouty deposits may eventually lead to destruction of the joints, resulting in deformity and a reduction of tendon strength. Spontaneous ruptures of the tibialis anterior, quadriceps, and Achilles tendons have been reported.5,6
In the acute stage of gout, MRI findings of the joint effusion and synovial thickening are nonspecific. As previously mentioned, radiographic findings of gout do not occur until the disease has been present for at least 6 or more years; hence a negative radiograph does not rule out the possibility of gout.3,7,8
Gouty tophi are mostly of low- or intermediate-signal intensity on T1W images and reveal an increase in signal intensity after intravenous administration of gadolinium. The imaging findings of tophi on T2W images are variable, but usually contain low-signal intensities.3 If on T2-weighted imaging, tophi have high signal intensity, it is believed that there is a lot of water in the joint. In cases of small foci with low-level enhancement, this may correspond to calcification, fibrosis, or hemosiderin within the tophi. Due to vascularization of the granulation tissue around the tophi, one might even see variable enhancement within the same tophus.7, 9-11
Calcium pyrophosphate dihydrate (CPPD)
In the 1960s, McCarty et al12,13 discovered new crystals in the synovial fluid, which were calcium pyrophosphate dihydrate. These were linear or punctuate deposits associated with certain diseases, such as chondrocalcinosis, pseudogout, and pyrophosphate arthropathies. Many diseases have been described in association with CPPD, such as osteoarthritis, diffuse idiopathic skeletal hyperostosis, hypertension, atherosclerosis, and diabetes mellitus, but there are no studies to link them.
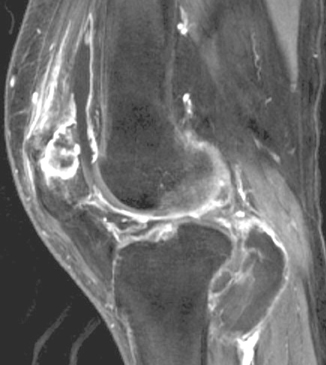
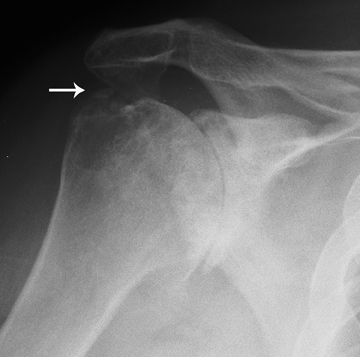
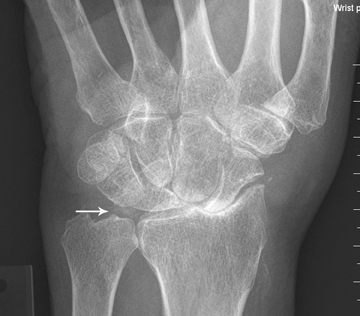
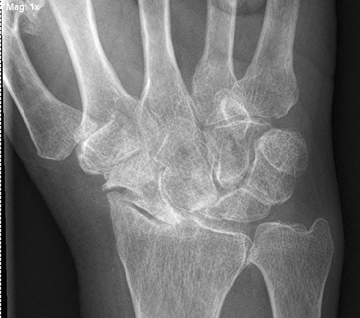
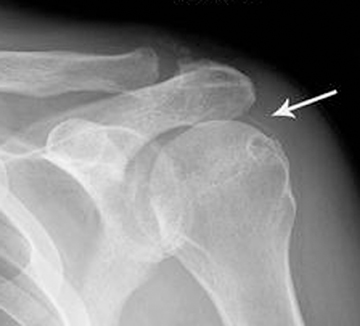
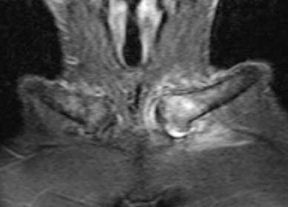
In chondrocalcinosis, the deposits are radiopaque seen as cartilage calcifications. Chondrocalcinosis is asymptomatic and often seen in elderly patients, especially in joints with hyaline cartilage and fibrocartilage, such as the knee and wrist. Pseudogout is very distinct from gout. The most common joints are knee and wrist. It is often seen in women >80 years old, which may be referred to as the disease of octogenarians. The shoulder is a non-weight-bearing joint and degenerative changes and narrowing and loss of articular cartilage seen in this joint with the absence of prior injury raises the possibility of CPPD (Figure 3). Calcification in the spinal ligaments specifically around the odontoid process is common in this disease (Figure 3). CPPD may be asymptomatic or cause arthritis with severe inflammation or may present as destructive joint disease, which may mimic neuropathic joint. The appearance of scapholunate advanced collapse (SLAC) in the wrist is an example of destructive features of pseudogout seen often in older age groups involved with CPPD (Figure 4). Focal calcifications around joints similar to tophaceous gout may be also seen in patients with pseudogout (Figure 5).
Interestingly, some gout patients have chondrocalcinosis; patients with hyperparathyroidism have an incidence of chondrocalcinosis. Other diseases, such as hemochromatosis and hypomagnesaemia, have an associated component with CPPD deposition.14
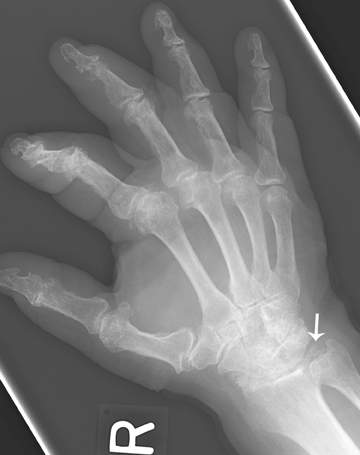
Metacarpophalangeal and femoropatellar joints are target joints in CPPD. Hemochromatosis creates characteristic prominent osteophytosis and hypertrophic degenerative appearance in the metacarpophalangeal joints (Figure 6). Hemochromatosis can cause chondrocalcinosis and inflammatory changes of the synovial joints (Figure 7). In cases of pseudogout, inorganic pyrophosphate levels in the synovial fluid are elevated. Metabolic diseases/factors associated with CPPD chondrocalcinosis15,16 include:
(A) Increased pyrophosphate (PPi) due to reduced PPi breakdown by alkaline phosphatase (ALP), which in turn can be due to:
- reduced ALP (eg, hypophosphatasia);
- the presence of ALP inhibitors, such as calcium (chronic hypercalcemia), copper (Wilson’s disease), and iron (hemochromatosis); and,
- deficiency of ALP cofactors, such as magnesium (chronic hypomagnesaemia) due to diet, chronic diarrhea, Gitelman syndrome, and Bartter’s disease.
(B) Increased PPi production through:
- chronic stimulation of adenylate cyclase, as in hyperparathyroidism;
- stimulation of PPi production (eg, by Vitamin A);
- increased calcium concentration;
- enhanced crystal nucleation (eg, by iron or copper); and,
- decreased crystal solubility, such as in hypomagnesaemia.
The joints with fibrocartilage and hyaline are more likely to develop CPPD; this makes the knees and wrists the most common sites.17 Radiographs of the knees will detect 90% of patients with calcifications in chondrocalcinosis, which are linear or punctuate in hyaline cartilage, but granular in fibrocartilage. Although CPPD is the most common cause of radiographic signs in chondrocalcinosis, both CPPD and calcium hydroxyapatite can be present. Interestingly, pyrophosphate arthropathy was described as pseudogout-like disease by Martel et al.18 It is a variant of osteoarthritis along with CPPD. There are also tophaceous pseudogout and tumoral CPPD, which are solitary space-occupying lesions, often seen in the digits (Figure 5).18,19
Some of the radiologic findings include joint effusion and soft-tissue swelling at the time of the pseudogout attacks, fractures in the surrounding bones; eg, tibia/carpal collapse due to radiocarpal osteoarthritis, also known as scapholunate advanced collapse of wrist; MCP joint involvement, and intervertebral disk calcification.19
Hydroxyapatite deposition diseases
These include hydroxyapatite (HA) and calcium orthophosphate dihydrate. Calcium hydroxyapatite comprises most of the bones and various other places in the body.
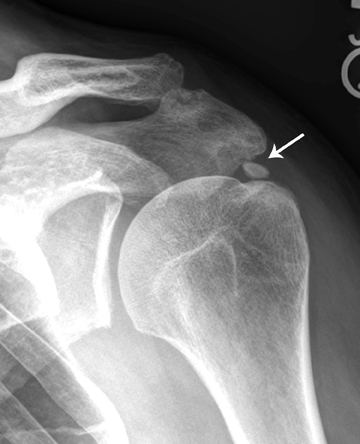
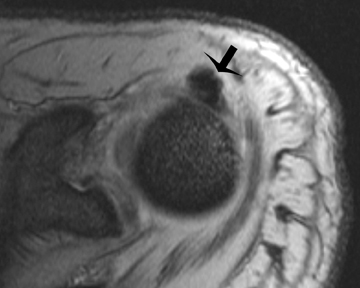
Deposits of HA very often are periarticular. These crystals are very small (70 – 300 nm) in diameter and hence, are difficult to identify. But as these crystals accumulate, larger forms can be stained and observed microscopically. HA deposits are often found in the shoulder in an area with minimal vascular supply (Figure 8). Hence it has been postulated that the HA are formed due to ischemia.20 Hypoxia in the critical area of the tendon may be an initiating event and could be followed by fibrocartilaginous metaplasia of the tendon, with propensity to calcify. Mononuclear cell infiltration with phagocytosis of calcium appears to follow and may correlate with symptoms.21
A distinguishing factor between CPPD and HA is that HA typically involves tendons, bursae, and periarticular tissue, whereas CPPD typically deposits in hyaline and fibrocartilage. Moreover, the deposition of HA may mimic an acute inflammation. HA depositions are often monoarticular and found in the shoulders, affecting both genders in middle age. They present acutely and are tender to palpation. Interestingly enough, HA depositions may also occur in asymptomatic patients observed radiographically. For those patients that are symptomatic, trauma or acutely increased use of the joint seem to be initiating factors.22
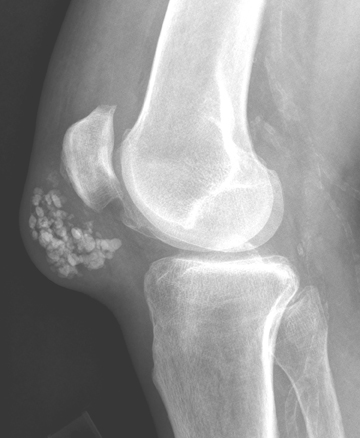
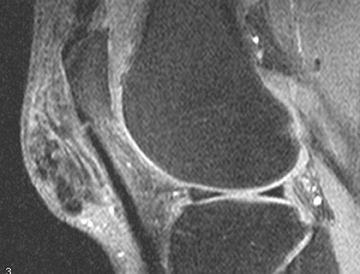
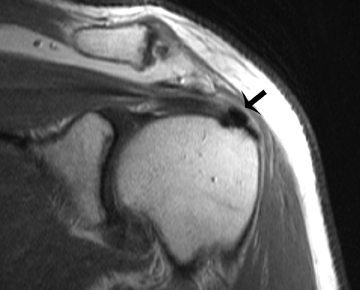
Pathologically HA deposits are granular calcified material in fibrous tissue in periarticular regions. They are also associated with necrosis. Radiographically they present differently depending on the age of the deposition (Figure 9). The early deposits have low density, are cloud-like, and have poorly defined shapes. On the other hand, older lesions are dense and opaque. Larger lesions cause cystic lesions. It has been radiographically shown that some of the patients undergoing an acute attack have in fact calcific material extruded into the adjacent joints, causing synovitis.23
Calcific tendinosis is most commonly seen in the shoulder in the distal supraspinatus tendon insertion at the greater tuberosity of the humerus. Less common locations may include tendons of infraspinatus, subscapularis, and deltoid, wrist, elbow, gluteus maximus, knee, and neck. Rarely erosion of bone adjacent to calcification at the insertion site of the tendons can be visualized. Hayes et al21 described 5 cases of calcific tendinosis with radiographic evidence of cortical bone erosions, including 2 at the pectoralis major insertion on the proximal humerus, 2 at the insertion of the gluteus maximus, and 1 at the insertion of the adductor magnus.
The right and more dominant rotator cuff is the most commonly involved location of calcification, specifically at the insertion of the supraspinatus tendon into the greater tuberosity. This makes the axial or external/internal rotation views important when looking for calcifications in this area. In other words, it is easier to see calcification in the supraspinatus tendon on external rotation and in the infraspinatus tendon on internal rotation. When crystals in the rotator cuff rupture, they cause synovitis. In addition, they accumulate in the more dependent area if they are in the joint, forming a zone of calcification and potentially predisposing the patient to rotator cuff tears.24 Interestingly, these foci of calcifications may go through resorption later on and can disappear on follow-up radiographs.
On MR imaging, HA deposits are of low signal. Moreover, there is often overlying inflammation and edema, which the radiologist may misinterpret as tenosynovitis or joint synovitis with infection or injury.24 At high calcium concentrations (above 30% to 40%) susceptibility effects and decreases in proton density dominate, leading to signal intensity loss.25 However, T1 shortening effects resulting in hyperintensity on T1-weighted images are also present. They have been attributed to surface interaction of protons with calcified tissue. At lower concentrations of calcium, T1 shortening effects dominate, resulting in isointensity or even hyperintensity.25,26 Gradient echo sequences best show these calcific foci.
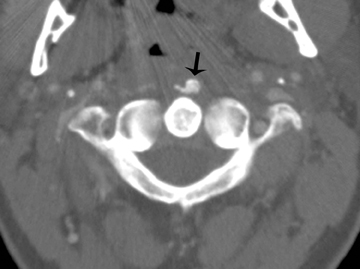
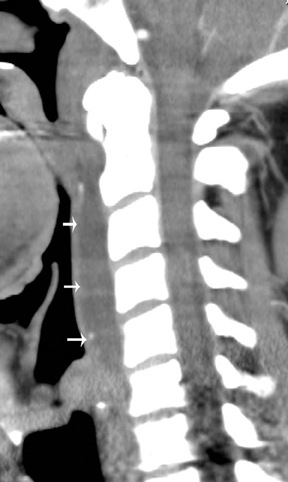
Acute retropharyngeal tendinitis (calcific tendinitis of the longus colli), initially described by Hartley in 1964, is an acute inflammatory condition of the longus colli tendon. It is related to calcium hydroxyapatite deposition in the superior oblique fibers of the longus colli muscle. Patients often present during their 3rd through 6th decade of life, most commonly with acute neck pain, stiffness, odynophagia, low-grade fever, and mild leukocytosis. The clinical presentation can be difficult to differentiate from a retropharyngeal abscess. Radiology can play a key role by identifying amorphous calcification in the proximal fibers of the longus colli, just inferior to the anterior arch of the atlas. Furthermore, radiology can be applied to the identification of prevertebral fluid collection/effusion, which is considered nearly pathognomonic of acute retropharyngeal abscess. The prevertebral fluid collections in this entity (Figure 9) tend to be smooth, linear/lenticular and do not contain an enhancing wall as one would expect with an abscess.27 Misdiagnoses, such as suppurative retropharyngeal infection, fracture dislocation of the cervical spine, myositis ossificans, and primary or metastatic neoplasia (eg, rhabdomyosarcoma), are frequently proposed by inexperienced radiologists.28
The correct imaging diagnosis is based on (1) the presence of pathognomonic calcification in the superior tendon fibers of the longus colli muscle, (2) the presence of fluid within the retropharyngeal space without associated enhancement around the effusion, (3) the absence of inflammatory retropharyngeal lymph nodes, (4) the absence of any bony destruction of the adjacent cervical vertebrae, and (5) recognition of the variability in the degree of tendinous calcium deposition, which may range from subtle to the more typical marked globular amorphous appearance.28
Conclusion
We reviewed the clinical and imaging features of 3 crystal deposition arthritides and illustrated their common and uncommon radiologic, CT, and MRI presentations.
References
- Tehranzadeh J. Musculoskeletal imaging cases. New York, NY: Mc Graw Hill; 2008.
- Nuki G. Gout. Medicine. 2006;34:417-423.
- Lagoutaris ED, Adams HB, DiDomenico LA, Rothenberg RJ. Longitudinal tears of both peroneal tendons associated with tophaceous gouty infiltration: A case report. J Foot Ankle Surgery. 2005;44: 222-224.
- Pfister AK, Schlarb CA, O’Neal JF. Vertebral erosion, paraplegia and spinal gout. AJR Am J Roentgenol. 1998;171:1430-431.
- Pascual E, Jovani V. Synovial fluid analysis. Best Pract Res Clin Rheumatol. 2005;19:371-386.
- Bond JR, Sim FH, Sundaram M. Radiologic case study: Gouty tophus involving the distal quadriceps tendon. Orthopedics. 2004;27:90-92.
- Miller LJ, Pruett SW, Losada R, et al. Tophaceous gout of the lumbar spine: MR findings. J Computer Assisted Tomography. 1996;20:1004-1005.
- Watt I, Middlemiss H. The radiology of gout. Clin Radiol. 1975;26:27-36.
- Chen CKH, Yeh LR, Pan HB, et al. Intra articular gouty tophi of the knee: CT and MRI imaging in 12 patients. Skeletal Radiology. 1999;28:75-80.
- Yu JS, Chung C, Recht M, et al. MR imaging of the tophaceous gout. AJR Am J Roengtgenol. 1997;168:523-527.
- Sheldon PJ, Forrester DM, Learch TJ. Imaging of intraarticular masses. Radiographics. 2005;25:105-119.
- McCarty DJ, Hollander JL. Identification of urate crystals in gouty synovial fluid. Ann Intern Med. 1961; 54:452-460.
- McCarty DJ, Kohn NN, Faires JS. The significance of calcium pyrophosphate crystals in synovial fluid of arthritis patients: the “pseudogout” syndrome. Ann Intern Med. 1962;56:711-737.
- Zitnan D, Sitaj S. Chondrocalcinosis polyarticularis (familiaris): Roentgenological and clinical analysis. Cesk Rentgenol. 1960;14:27-34.
- Timms AE, Zhang Y, Russell RG, Brown MA. Genetic studies of disorders of calcium crystal deposition. Rheumatology. 2002;41:725-729.
- Scotchford CA, Greenwald S, Ali SY. Calcium phosphate crystal distribution in the superficial zone of human femoral head articular cartilage. J Anat. 1992;181:293-300.
- Doherty M, Dieppe P, Watt I. Low incidence of calcium pyrophosphate dihydrate crystal deposition in rheumatoid arthritis, with modification of radiographic featuers in coexistent disease. Arthritis Rheum. 1984; 27:1002-1009.
- Martel W, Champion CK, Thompson GR, et al. A roentgenologically distinctive arthropathy in some patients with the psuedogout syndrome. Am J Roentgenol Radium Ther Nucl Med. 1970;109:587-605.
- Steinbach LS, Resnick D. Calcium pyrophosphate dihydrate crystal deposition disease revisited. Radiology. 1996;200:1-9.
- Giachelli CM. Inducers and inhibitors of biomineralization: Lessons from pathological calcification. Orthod Craniofacial Res. 2005;8:229-231.
- Gondos B. Observations on periarthritis calcarea. Am J Roentgenol Radium Ther Nucl Med. 1957;77:93-108.
- Bui-Mansfield LT, Moak M. Magnetic resonance appearance of bone marrow edema associated with hydroxylapatite deposition disease without cortical erosion. J Comput Assist Tomogr. 2005;29:103-107.
- Jim YF, Hsu HC, Chang CY, et al. Coexistence of calcific tendonitis and rotator cuff tear: An arthrographic study. Skeletal Radiol. 1993;22: 183-185.
- Leitersdorf E, Reshef A, Meiner V, et al. Frameshift and splice-junction mutations in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis in Jews of Moroccan origin. J Clin Invest. 1993;91:2488-2496.
- Zubler, C, Mengiardi B, Schmid MR, et al. MR arthrography in calcific tendinitis of the shoulder: Diagnostic performance and pitfalls. Eur Radiol. 2007;17:1603-1610.
- Wasserman E, Richman A, Erdag N, Weiss R. Acute calcific prevertebral tendonitis of the longus colli muscle. Applied Radiology. 2009;38:169.
- Offiah, CE, Hall E. Acute calcific tendinitis of the longus colli muscle: Spectrum of CT appearances and anatomical correlation. Br J Radiol. 2009;82:117-121.
- Farpour F, Phan SJ, Burns J, Tehranzadeh J. Enhanced MR imaging of the shoulder, and sternoclavicular and acromioclavicular joint arthritis in primary hemochromatosis. Rheumatol Int. 2011;31:395-398. Epub 2009 Oct 14.
Related Articles
Citation
Imaging of crystal deposition disease. Appl Radiol.
August 6, 2012