Complications of abdominal transplantation at CT and MRI
Images



Dr. Yeh is an Assistant Professor of Abdominal Imaging and Dr. Coakley is an Associate Professor and the Chief of Abdominal Imaging in the Department of Radiology, University of California, San Francisco, CA.
In the United States, more than 300,000 organ transplants have been performed, more than 250,000 of which were abdominal organs. 1 The number of transplants performed each year continues to rise, 1 and the radiologist plays a key role in monitoring and diagnosing disease in this special patient population. This article will discuss radiologically evident complications associated with solid abdominal organ transplants.
Kidney transplants
The most commonly transplanted abdominal organ is the kidney. More than 14,000 renal transplants were performed in the United States in 2003, and 86,000 patients are on the kidney transplant waiting list. 1 Common indications for renal transplants are medical renal disease (36%), diabetic nephropathy (21%), and hypertensive nephropathy (15%). 1 While renal transplantation is not a life-saving procedure, it greatly improves quality-of-life and is more cost-effective in the long term compared with chronic hemodialysis.
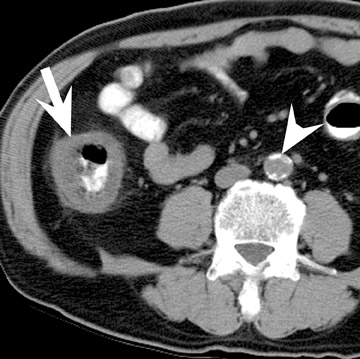
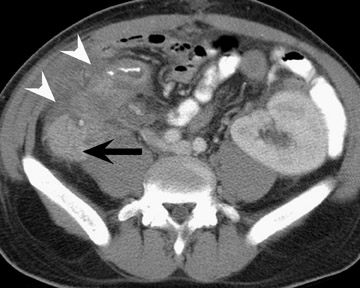
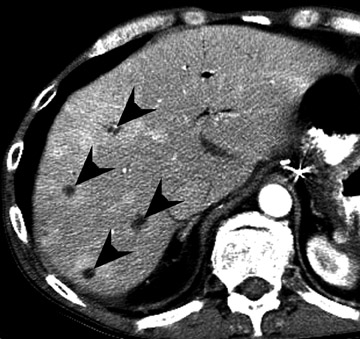
Assessment of a renal transplant recipient must also include evaluation for complications of the patient's underlying disease. If the patient has diabetes or continues to smoke, atherosclerosis may persist or progress. Bowel paresis or ischemia and arterial stenoses may still occur and must not be overlooked as a cause of abdominal pain (Figure 1). Furthermore, renal allograft recipients may have ongoing sequelae from prior peritoneal dialysis, such as bowel adhesions or sclerosing peritonitis, 2 a thickening of the peritoneum that may be associated with prior peritoneal infection (Figure 2).
The typical renal allograft is placed in an extraperitoneal location protected posterolaterally by the iliac bone. The donor renal artery and vein are anastomosed to the recipient external or internal iliac vessels, and the ureter is tunneled into the bladder near the trigone to minimize reflux but may be anastomosed elsewhere in the bladder. 3 Two kidneys may be transplanted en bloc into an adult iliac fossa if the donor is a child.
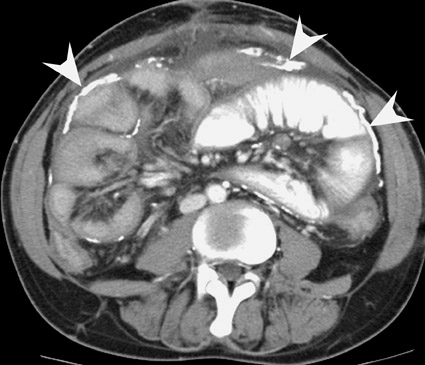
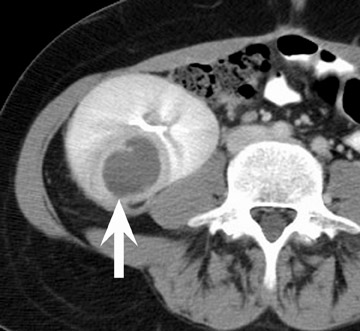
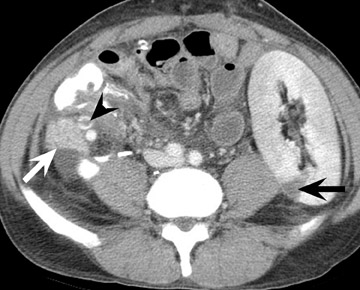
In the first few weeks after transplantation, perinephric fluid collections may be seen, usually in the extraperitoneal space (Figure 3). These fluid collections tend to displace adjacent structures such as the bowel, and may surround the bladder or retroperitoneal vessels. A high-attenuation collection may be due to hematoma, possibly indicating a leaking vascular anastomosis, requiring urgent surgical revision. Careful evaluation for active extravasation of intravenous contrast is imperative. If the collection is of fluid density, delayed computed tomographic (CT) images revealing filling of this collection with dense contrast material at CT is diagnostic of a urinoma secondary to a leak or rupture of the ureter. Rim-enhancing fluid collections, especially if gas-containing, suggest abscess, and percutaneous or surgical drainage should be performed.
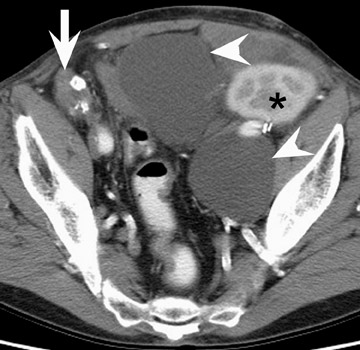
More benign collections, such as seromas or lymphoceles, may also occur, but these collections may also become secondarily infected. In general, seromas tend to shrink spontaneously with time, while lymphoceles can grow. Lymphoceles are particularly common around renal transplants as compared with other abdominal transplants, and occur in up to 20% of patients 4 (Figure 3). The development of lymphoceles is due to both disruption of lymphatic channels draining the kidney during organ procurement and the posttransplantation medical regimen. 4
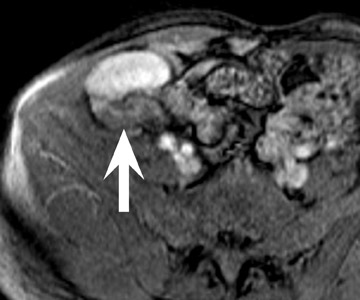
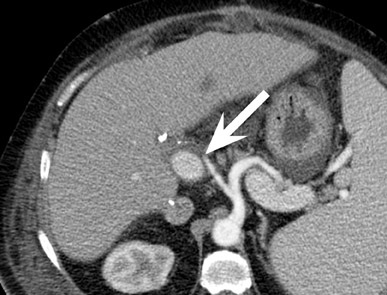
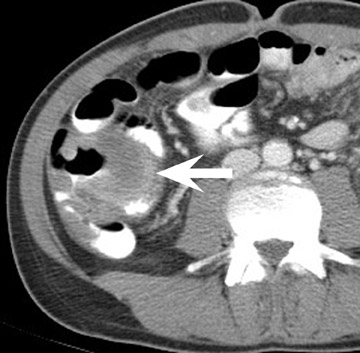
Renal vascular complications are feared because they may cause loss of the allograft. CT and magnetic resonance (MR) angiography may be helpful to delineate patency of the renal arteries and veins. The most common vascular complication in renal transplants is renal artery stenosis, which occurs in 1% to 23% of patients. 5 This complication most commonly occurs soon after transplantation but may occur several years afterwards. Stenosis is most common at the anastomotic site and is often amenable to dilatation by percutaneous angioplasty. 6 Renal parenchymal abnormalities, such as wedge-shaped infarcts, poor perfusion on venous-phase imaging or on prolonged nephrogram, may be also be associated with renal artery stenosis (Figure 4).
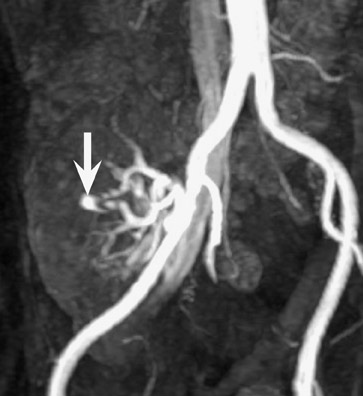
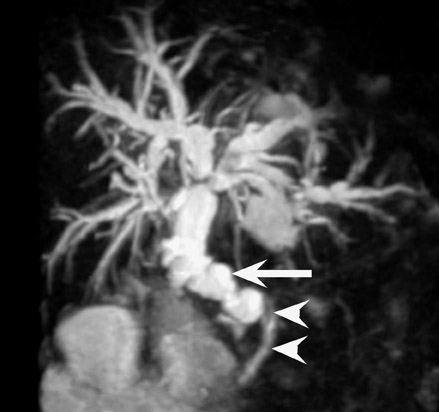
Arteriovenous fistula and pseudoaneurysms may occur in a renal allograft as a result of prior percutaneous biopsy and may cause hematuria, hypertension, or decreased renal function. 7 Doppler ultrasound (US) may show a characteristic enlarged vascular space with, in the case of an arteriovenous fistula, decreased resistance of the arterial waveform, and arterialization of the venous waveform. At CT or MR angiography (Figure 5), a prominent vascular cavity may be seen, possibly with enlargement of the draining renal vein. Although smaller lesions may be monitored closely for progression or resolution, larger fistulae may be treated effectively with transarterial embolization. 8
The ureter of a renal allograft becomes strictured in up to 10% of patients, 9,10 most commonly due to insufficient blood supply. In more severe cases, ureteral necrosis and urine leak may develop, particularly in patients undergoing high-dose steroid treatment. Ureteral stent placement or balloon dilatation is helpful to relieve obstruction, 11,12 but ureteral revision may be required.
Renal parenchymal complications, such as infarcts and pyelonephritis, may be seen in the early posttransplant period (Figure 6). Pyelonephritis may also occur some time after the time of transplantation, and the risk of infection may be increased with the presence of ureteral reflux. Ill-defined parenchymal perfusion abnormalities, perinephric fat stranding, and urothelial thickening of the renal pelvis and ureter are signs of possible infection. Although the common pathogens are gram-negative bacteria, Candida and other fungal infections may occur.
CT and MR evaluation for possible renal transplant rejection is not reliable, 13 and percutaneous biopsy is required for accurate diagnosis. Imaging abnormalities for rejection are nonspecific, and include decreased perfusion or swelling of the allograft, thickening of the urothelium, and loss of corticomedullary differentiation. 13
When renal allograft failure occurs, the allograft is usually left in place. The appearance of this allograft may range from that of a normal-appearing kidney to an atrophic or hydronephrotic kidney to an amorphous heavily calcified mass (Figure 3). 14 Failed allografts should not be mistaken for pelvic lymphadenopathy, tumor, or contrast-filled bowel. 15
Liver transplants
More than 5000 patients receive liver transplants each year, and the demand for liver transplantation is increasing, particularly with the rising prevalence of hepatitis C. While the most common indication for liver transplantation is liver failure, patients with hepatocellular carcinoma and small tumor burden may be cured by liver transplantation. Currently, 5000 patients die annually on the waiting list for a liver. 1 In patients who receive a liver transplant, graft survival is approximately 80% at 3 years. 1
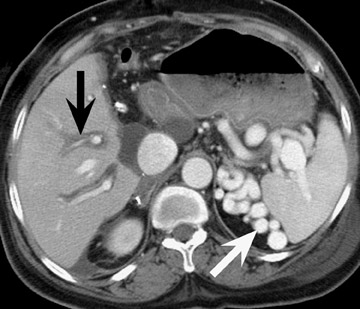
Liver transplantation surgery is complex and requires anastomosis of the donor to recipient bile duct, inferior vena cava, hepatic artery, and portal vein. Unlike kidney and pancreatic transplantation, liver transplantation requires resection of the native organ prior to transplantation of the donor organ. Stigmata of prior cirrhosis, such as varices and splenomegaly, often persist after successful liver transplantation (Figure 7). Furthermore, periportal edema is a common and persistent finding that does not imply pathology (Figure 8).
Biliary complications occur in up to 30% of liver transplant recipients 16 and are the most common structural complication of liver transplantation. Fluid collections in the abdomen in the immediate posttransplant period are most commonly bilomas, which frequently appear as fluid- density or fluid-signal structures around the liver, particularly along the cut edge of the liver in patients with partial liver transplants. Although many of these collections resolve spontaneously, persistent or enlarging collections may indicate a significant leak and may require biliary tract stenting or percutaneous drainage. 17,18 Bile duct leak is more common with bili-enteric anastomosis than with duct to duct anastomosis, 19 and more common in living donor liver transplants than in cadaveric liver transplants. Evaluation of biliary leak may be performed using percutaneous or retrograde cholangiography, but alternative methods include scintigraphy or excretory CT or MR cholangiography. 20
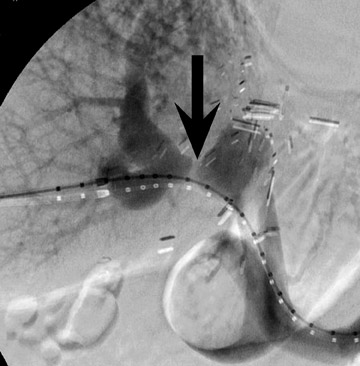
Biliary stenosis may result in elevation of liver enzymes, and noninvasive imaging with MR or CT cholangiography may be performed to assess severity (Figure 9). Nonocclusive stenoses may be treated with percutaneous or endoscopic stenting and balloon dilatation, 21 but occasionally surgical revision may be necessary. Most biliary stenoses occur at the anastomosis site, but intrahepatic biliary stenosis may occur in 10% of 17,18,22 and may be associated with hepatic arterial thrombosis, rejection, or cholangitis.
An important point to realize is that occasionally bile ducts in the liver allograft may need to be sacrificed and ligated if they do not connect to the main duct. These ligated ducts are expected to dilate over time, and the associated liver parenchyma will atrophy. Unless these ducts are known to be infected, percutaneous drains must not be placed into these dilated ducts, unless a subsequent internal bilioenteric anastomosis is feasible, otherwise subsequent removal of the percutaneous drain may not be possible.
Evaluation of vascular anastomoses is important for liver transplant recipients; ideally both an arterial and portal venous phase examination should be performed at CT or MRI. The most common vascular complication of liver transplants is hepatic artery thrombosis or stenosis, which may occur in up to 15% of recipients 23-25 (Figure 10). The outcome of hepatic artery thrombosis is variable, and may range from elevated liver enzymes to biliary stricture and necrosis to fulminant hepatic necrosis and loss of the graft. Intrahepatic bilomas and abscesses due to hepatic artery thrombosis may be successfully treated with percutaneous drainage. 26 CT and MR angiography are both useful modalities for evaluating hepatic arterial complications. 27-29
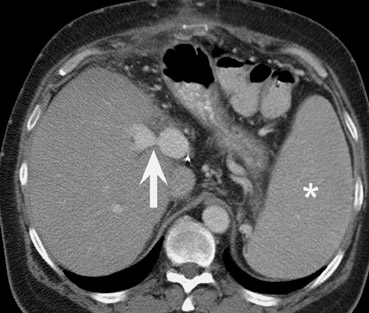
Stenoses or thromboses of the venous structures are less common complications of liver transplants. Portal vein compromise may result in ascites, variceal bleeding, and liver failure. Both CT and MR are useful modalities to assess for severity of portal vein pathology (Figure 11). It should be noted that the presence of varices, such as a splenorenal shunt or perigastric varices, is commonly seen after liver transplantation: The mere presence of varices does not imply portal venous hypertension (Figures 7 and 8).
Liver transplant recipients are at risk of recurrence of their pretransplant disease. In particular, since hepatitis C is the most common indication for liver transplantation in the western world, progression of viral disease in the allograft may occur. Furthermore, in patients with hepatocellular carcinoma or other intrahepatic malignancy prior to transplantation, tumor recurrence may occur. The most common sites of hepatocellular carcinoma recurrence are the lung and the liver 30 (Figure 12).
Pancreas transplantsPancreatic transplantations are performed primarily in patients with diabetes, and are usually placed in tandem with, or after, a kidney transplantion. 31 The main motivation for pancreatic transplantation is to address the endocrine deficiency of patients with diabetes who cannot produce sufficient insulin. While pancreatic transplantation is not a life-saving procedure, it may prevent long-term complications of diabetes, including neuropathy, blindness, and renal failure.
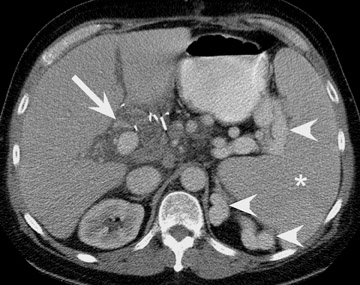
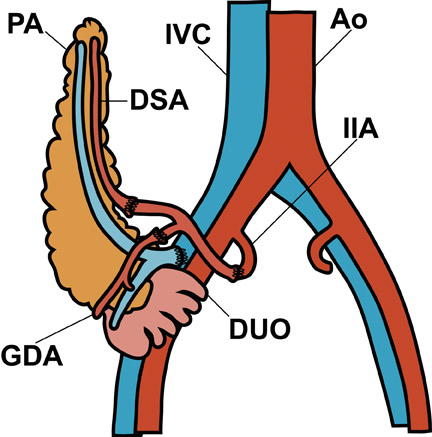
Visual identification of the pancreatic allograft is challenging if the radiologist is unaware of the anatomy of pancreatic transplants. The head of the pancreatic allograft is typically in the right pelvis, with the tail extending superiorly along the right paracolic gutter. Multiple arterial and venous anastomoses are typically performed to join the allograft vessels with the recipient iliac vessels (Figure 13). The donor duodenum, which drains the pancreatic duct, is anastomosed to either the bladder or the ileum of the recipent. 32 Patients with duodenal-to-bladder drainage of the pancreas allograft are at slightly higher risk for urologic complications and dehydration. 32 Peripancreatic fluid collections are very common after pancreatic transplantation, and some degree of allograft pancreatitis is always noted clinically (Figure 14). Visualization of the pancreatic allograft at MRI is often best with fat-saturated T1-weighted sequences after gadolinium administration.
Vascular thombosis is more common in pancreatic transplants than in renal and hepatic allografts, and may occur in up to 30% of patients. 33-35 This predisposition may be due to intravascular stasis caused by the relatively low volume of blood passing through the pancreatic parenchyma and due to the discordant caliber of the inflow arteries (splenic and gastroduodenal arteries) relative to those of the pancreatic parenchymal arteries. Furthermore, pancreatitis in the allograft, medical therapy, and prolonged ischemia time may predispose recipients of pancreatic allografts to vascular thrombosis. 34 Regardless of the appearance of the pancreatic vessels, the pancreatic parenchyma must be assessed for enhancement to identify possible pancreatic necrosis (Figure 15). A pitfall in evaluation of pelvic veins by CT and MRI is iliac vein pseudo-thrombosis, which is a laminar flow phenomenon caused by more rapidly enhanced venous return from the renal allograft than from the lower extremities. This early venous return from the renal allograft creates the impression of ipsilateral partial iliac vein thrombosis, as opacified blood flows adjacent to unopacified blood, or creates the impression of contralateral iliac vein thrombosis because the iliac vein opacifies sooner on the renal allograft side than on the other side. 36
A new technique for pancreatic transplantation is the injection of purified insulin-producing islets into the portal vein rather than the transplantation of the entire donor pancreas. These injected pancreatic islets embolize into the liver parenchyma and function to produce insulin. In patients receiving such transplants, a heterogeneous appearance of the liver parenchyma may be seen soon after transplantation but, subsequently, its appearance returns to normal.
General complications of transplantation
Posttransplantation lymphoproliferative disorder (PTLD) is a feared complication that may occur with any type of allogenic transplant and affects 2% to 5% of solid-organ transplant patients. 37 The severity of PTLD may range from local lymphatic tissue hyperplasia to aggressive systemic lymphoma. Risk factors for PTLD include an Epstein-Barr virus-negative recipient who receives an Epstein-Barr virus-positive allograft, history of intensive immunosuppressive therapy, and hepatitis C. Patients requiring higher baseline immunosuppressive therapy, such as heart and lung transplant patients, have a higher risk (up to 15%) for developing PTLD. 38,39 Abdominal PTLD commonly involves extranodal sites, such as the bowel and liver (Figure 16), but nodal involvement occurs in approximately 20%. 37 While PTLD most commonly occurs within the first year after transplantation, it may occur remote from the time of transplantation.
The imaging appearance of PTLD closely mimics that of lymphoma. Focal masses tend to be homogeneous and hypovascular and tend to displace rather than occlude the bowel, vessels, and ureters. Also, PTLD may appear as an infiltrating hilar mass, both in renal and hepatic allografts 13,37,40 (Figure 17). Infiltrating tumors may also occur within a transplant organ and may be difficult to appreciate at imaging. Core or open biopsy is generally required for diagnosis of PTLD. Patients with PTLD are treated with a decrease in their immunosuppressive therapy, and if PTLD is localized to the allograft, resection may be curative. More aggressive disease may require chemotherapy or radiation therapy. The overall 5-year survival rate for PTLD is 59%, and a poorer prognosis is associated with onset within the first year after transplantation. 41
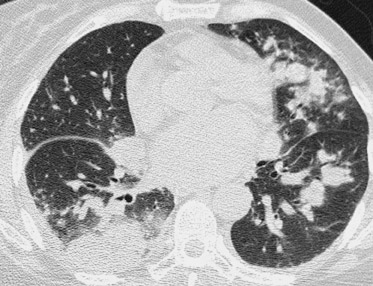
Due to immunosuppressive therapy, transplant patients are also at risk for nosocomial and opportunistic infections. Pulmonary infections are common and are most typically bacterial, but atypical pathogens (including tuberculosis, aspergillus, cytomegalovirus, and coccidiomycosis) should also be suspected when patients do not respond to antibiotic therapy 42 (Figure 18). Likewise, focal infections of the allograft or colon may be due to atypical organisms (Figure 19).
For all abdominal transplants, evaluation for possible rejection remains a difficult diagnosis that largely depends on core or open biopsy diagnosis.
Conclusion
As the population of patients with abdominal transplantation grows, radiologists will increasingly be called upon to assess these patients for possible complications. Knowledge of the anatomy and the common complications in this special population is crucial for making appropriate diagnoses.
Related Articles
Citation
Complications of abdominal transplantation at CT and MRI. Appl Radiol.
May 4, 2005