Combination Therapy Improves Efficacy in Widespread Prostate Metastatic Disease
Images
By: Nat Lenzo, MMed MSc(Oncol), EMBA, FRACP FAANMS
Brought to you by Telix.
CASE SUMMARY
A 79-year-old man with prostate cancer previously treated in 1997 with radical prostatectomy and subsequently treated with hormone therapy (ADT, abiraterone), chemotherapy (docetaxel, cabazitaxel), and strontium for metastatic bone disease presented in July 2017 with rising PSA levels.
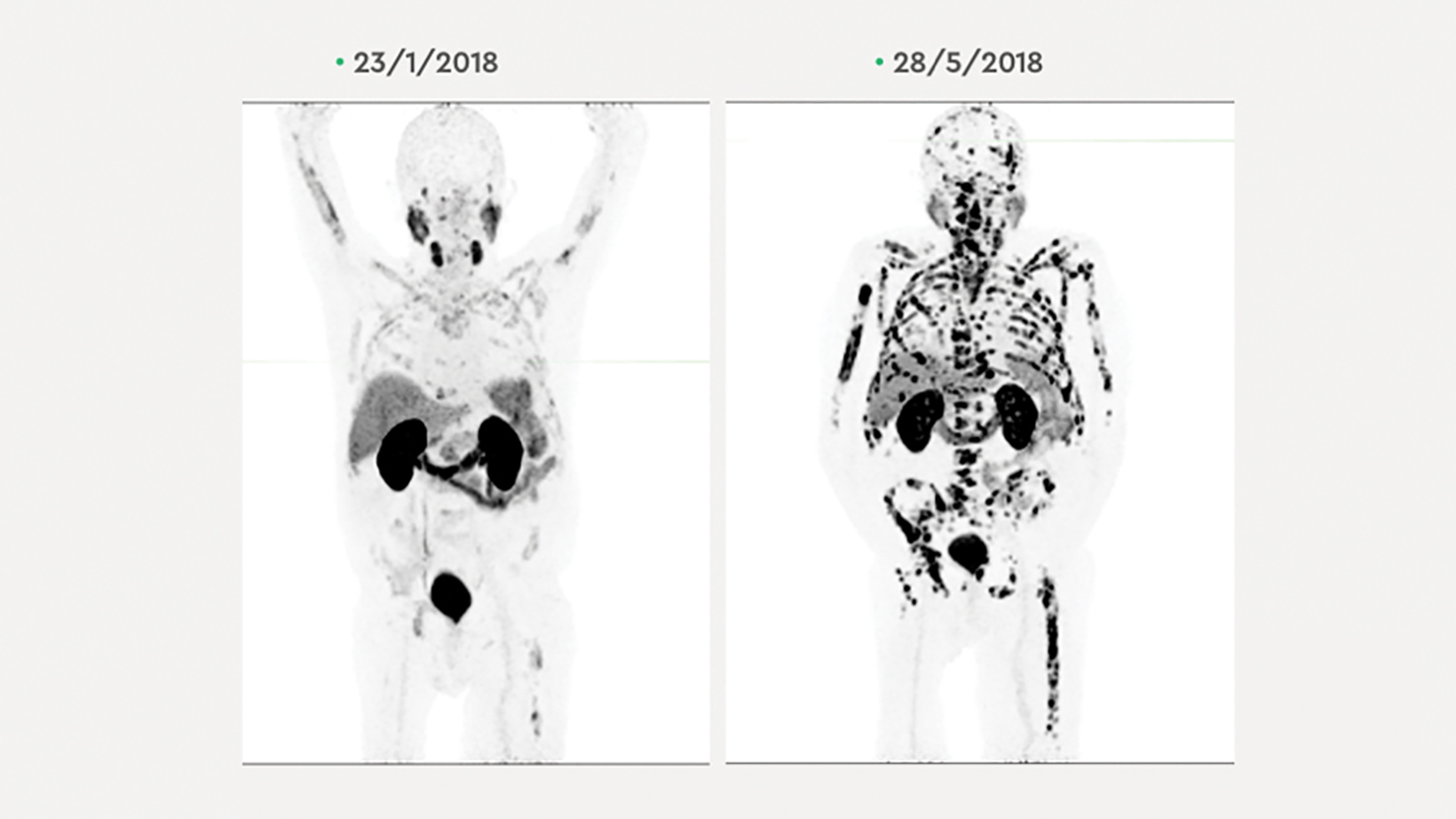
The patient’s PSA levels rose from 764 ug/L on July 4, 2017, to 1,722 ug/L on Sept. 5, 2017, 1,950 ug/L on Sept. 25, 2017, to 2,481 ug/L on Nov.1, 2017, when gallium-68 ( 68 Ga) PSMA-11 PET/CT scanning was performed.
IMAGING FINDINGS
The 68 Ga-PSMA-11 PET/CT demonstrated widespread bone and bone marrow metastases. Liver, lung, and nodal metastases were also noted. The patient also had mildly low hemoglobin (Hb 118 g/L) and a high platelet count (449) with elevated lactic dehydrogenase (LDH 526 U/L). The patient had known renal impairment (eGFR 40 ml/min). The patient’s ECOG status was 1.
After written informed consent and Therapeutic Goods Administration (Australia) Special Access Scheme approval, the patient was treated as per institutional guidelines with 6.12 GBq lutetium-177 ( 177 Lu) PSMA on Nov. 2, 2017. His PSA level dropped to 1,282 ug/L on Nov. 28, 2017, and to 637 ug/L on Dec. 18, 2017. Hemoglobin remained low (119 g/L) and platelets (324) and LDH (422 U/L) remained high. A second dose of 177 Lu PSMA was administered on Dec. 21, 2017, further lowering the patient’s PSA to 239 ug/L on Jan. 16, 2018, and even lower, to 54.4 ug/L, on Feb. 22, 2018. The patient’s hemoglobin (129 g/L), platelets (276) and LDH (361 U/L) also began to normalize.
A 68 Ga-PSMA-11 PET/CT scan subsequent to the second 177 Lu PSMA treatment showed resolution of liver, lung and nodal disease; however, avid bone disease persisted. The patient’s PSA level was monitored for 4 months, during which time it rose to 1,295 ug/L by May 24, 2018.
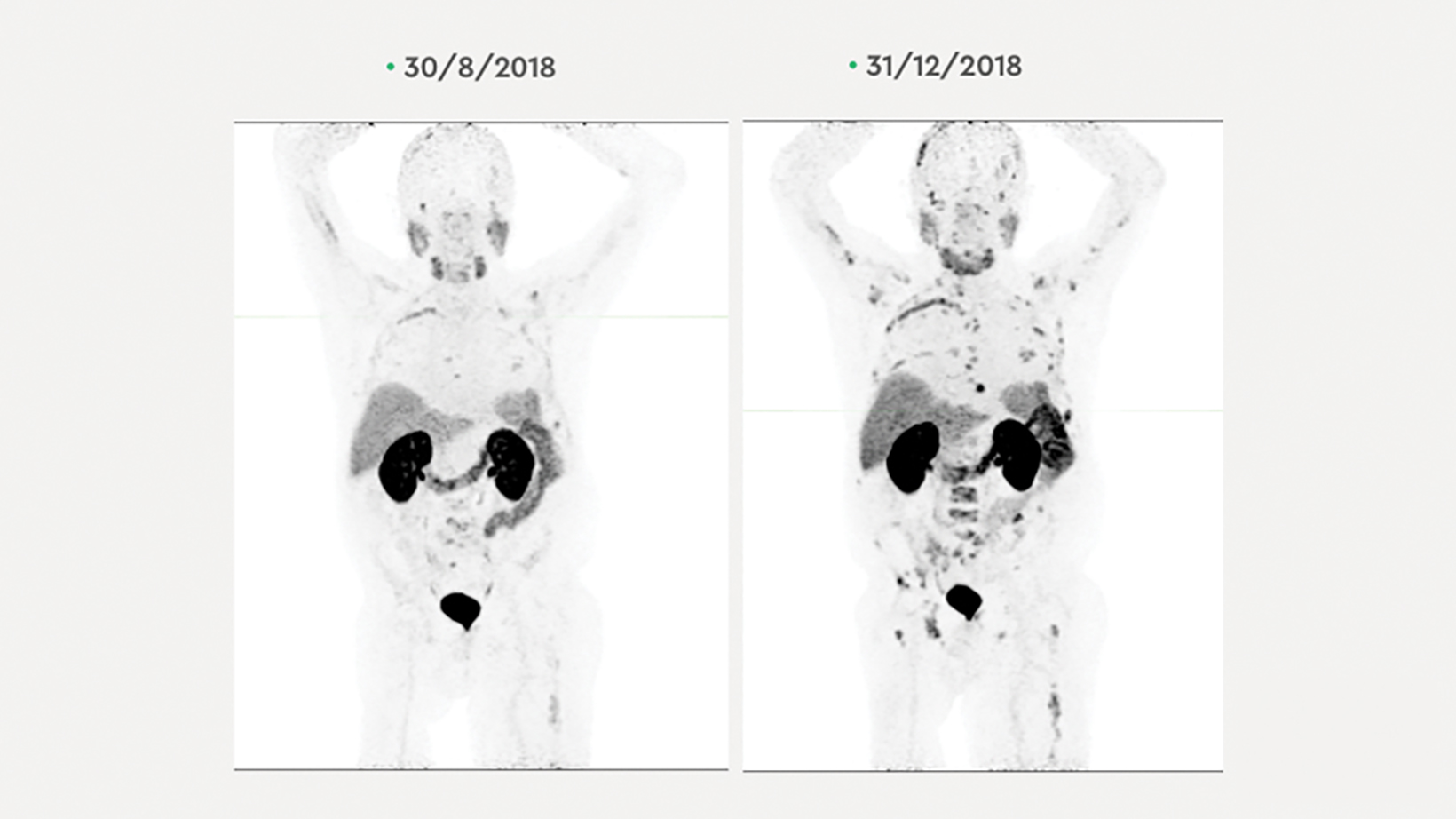
A68Ga-PSMA-11 PET/CT scan on May 28, 2018, demonstrated progression of widespread bone disease and recurrence of metastatic disease in the liver, right hilum and mediastinum. (Figure 1) The patient underwent 2 cycles of 177 Lu PSMA: 6.62 GBq on June 1, 2018, and 7.14 GBq on July 27, 2018, accompanied by daily doses of enzalutamide 160 mg. The patient’s PSA level dropped to 149 ug/L by Aug. 22, 2018, when another 68 Ga-PSMA-11 PET/CT scan showed resolution of nearly all metastases and minimal PSMA-avid bone disease.
The patient continued 177 Lu PSMA treatment with 7.63 GBq on Oct. 4. 2018, and 7.6 GBq on Nov. 30, 2018, with enzalutamide 160 mg daily (Figure 2).
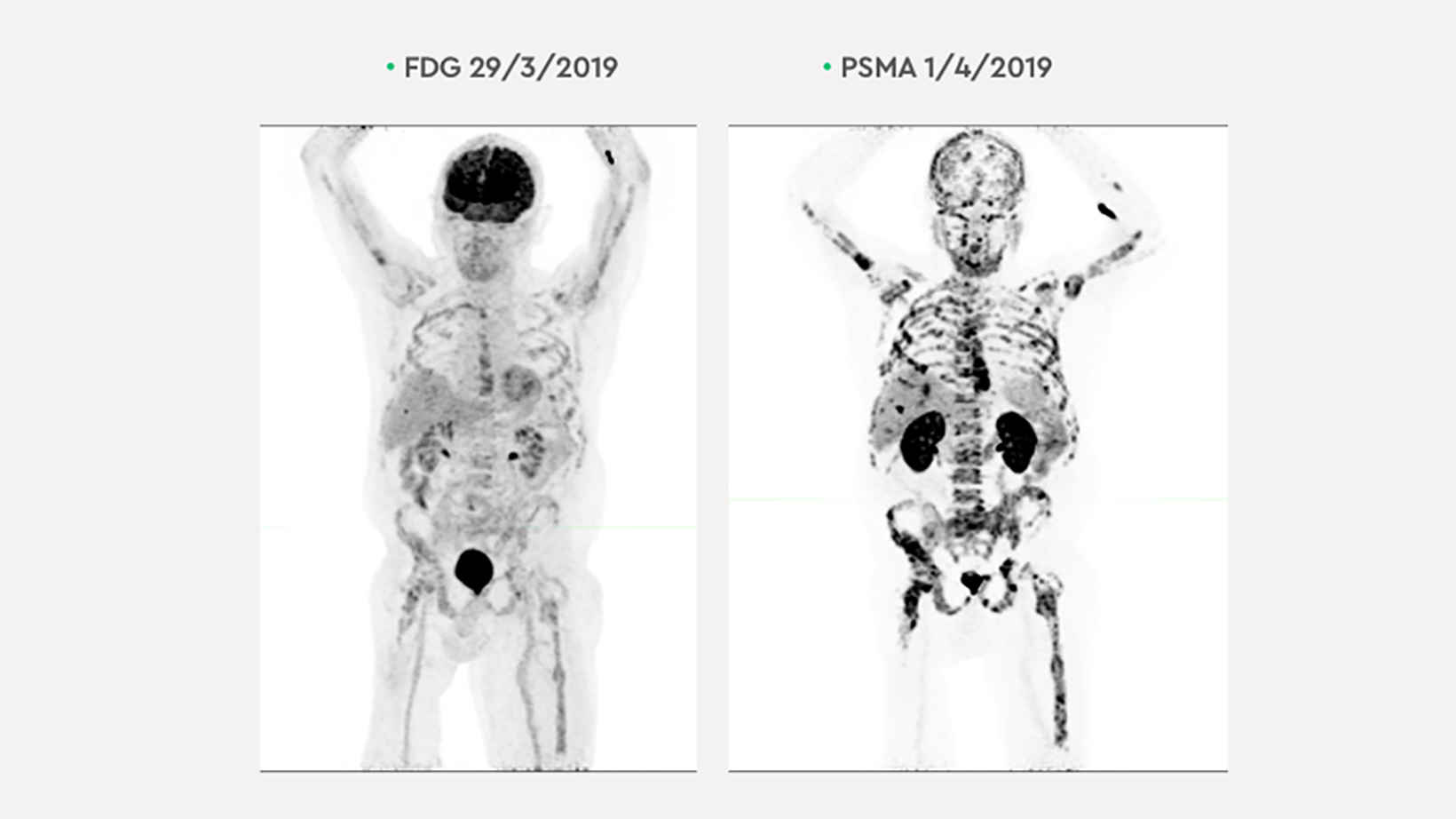
The patient’s PSA levels began rising again in early 2019, reaching 2,030 ug/L by Feb. 4, indicating disease progression. The patient did not respond to 7 GBq 177 Lu PSMA with capecitabine 500 mg bd for 10 days. FDG PET and PSMA PET/CT exams demonstrated concordant disease; however, the 68Ga-PSMA-11 PET/CT depicted more extensive disease (Figure 3). The patient’s hemoglobin was very low (98 g/l); platelets and LDH were normal. Renal function remained stable.
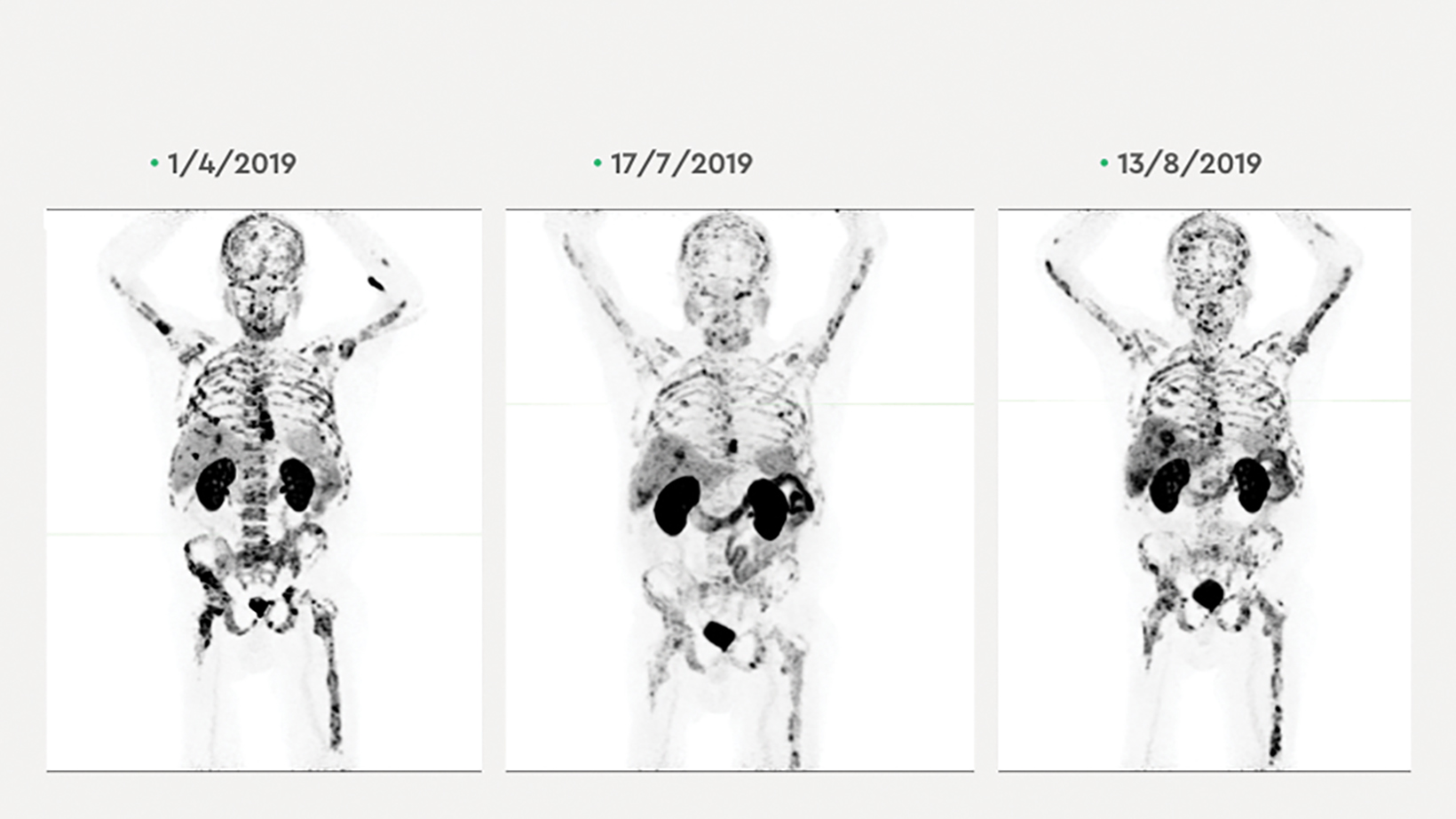
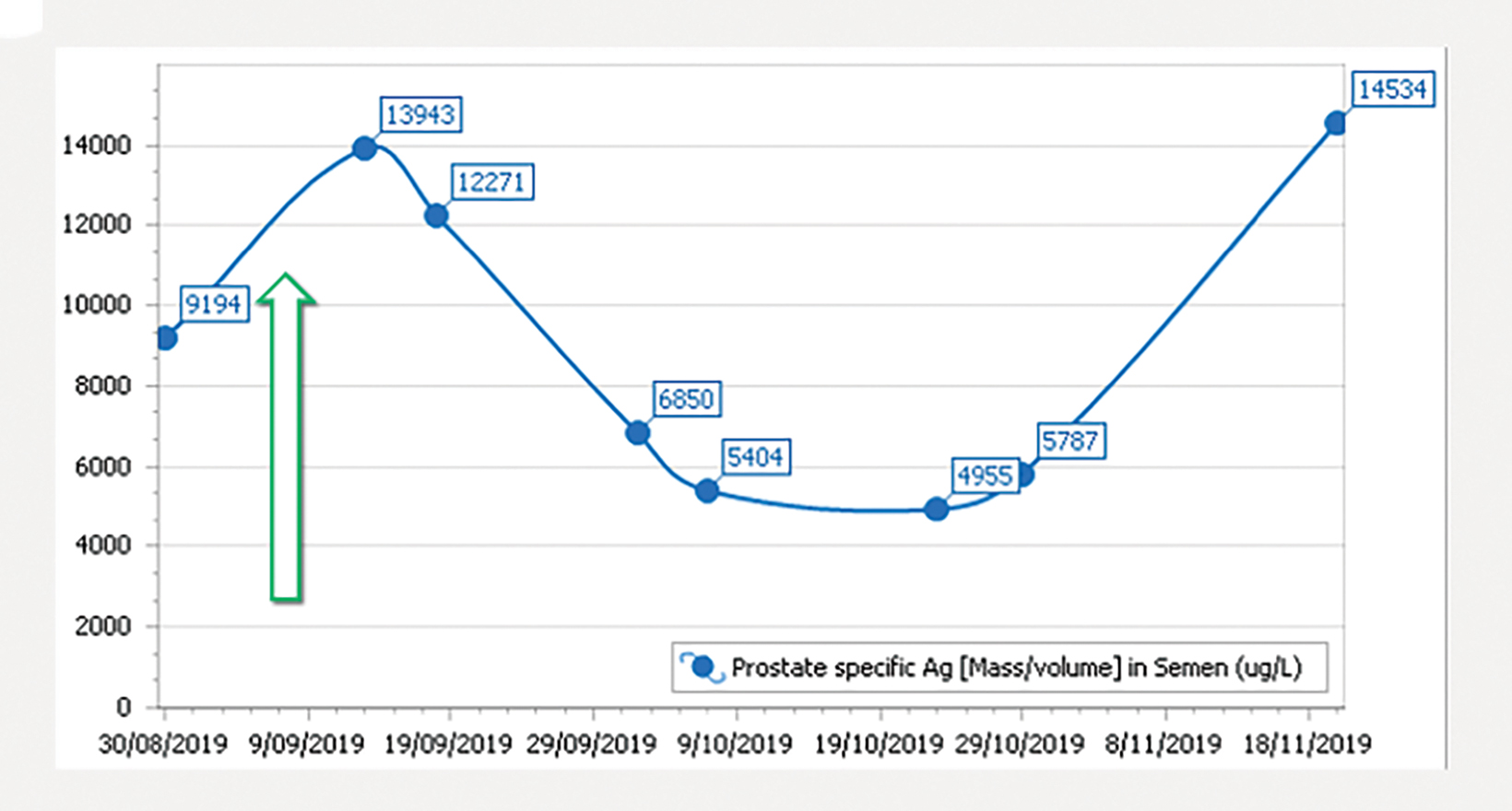
The patient underwent 2 courses of actinium-225 ( 225 Ac) PSMA, 5.63 MBq on April 24, 2019, and 5.8 MBq on June 15, 2019. After an initial drop to 88.8 ug/L following the first course, his PSA level rose to 2,829 ug/L on June 7, 2019, and then fell slightly to 2,332 ug/L after the second course. The PSA level then rose again to 6,895 ug/L on Aug. 22, 2019 (Figure 4). A third course of 225 Ac PSMA, 7.15 MBq, was then initiated on Sep. 6, 2019, in combination with the radiosensitizer idronoxil (Veyonda). 1 His PSA fell from a peak of 13,943 ug/L in September 2019, just after 225 Ac PSMA and idronoxil administration to a nadir of 4,955 ug/L in October 2019, 6 weeks after combination radioligand therapy (Figure 5). The patient developed symptomatic xerostomia following 3 cycles of 225 Ac PSMA. 2
The patient remained essentially symptom-free until late October 2019, when his health began declining. The patient actively withdrew from treatment and he died in late November 2019.
DISCUSSION
Over 2 years, the patient received 7 cycles of 177 Lu PSMA with a total administered activity of 49.3 GBq, and 3 cycles of 225 Ac PSMA with a total administered activity of 18.6 MBq. There was no additional renal toxicity seen; only self-limiting hematological toxicity was seen following treatments.
Both radioligand therapies were well tolerated by the patient, who demonstrated other comorbidities, including renal impairment, and could be repeated safely and successfully. As a result, the patient remained nearly symptom free until end-of-life.
CONCLUSION
Combination therapies such as 177 Lu PSMA and 225 Ac PSMA can safely be administered without short- to medium-term nephrotoxicity and can improve outcomes in elderly patients with advanced metastatic castrate-resistant prostate cancer. FDG PET may be useful for treatment planning, while 68 Ga-PSMA-11 PET/CT can be utilized to monitor treatment efficacy. 177 Lu PSMA is a promising treatment for patients with advanced metastatic prostate cancer even in patients with mild renal impairment. 3 225 Ac PSMA may have a role in salvage therapy post 177 Lu PSMA to improve progression-free and overall survival. 2 Combination treatments including radiosensitizers and novel anti-androgen drugs can be safely administered with radioligand therapies and may improve efficacy of treatment. 1,3 68 Ga-PSMA-11 PET/CT-directed targeted radioligand therapies can induce periods of remission and improve quality of life in advanced metastatic prostate cancer.
Related Articles
References
Citation
Combination Therapy Improves Efficacy in Widespread Prostate Metastatic Disease. Appl Radiol.
November 6, 2021