Blue Earth Diagnostics, Siemens Healthineers to Collaborate on AI-Based Tools for Prostate PET Imaging
Images
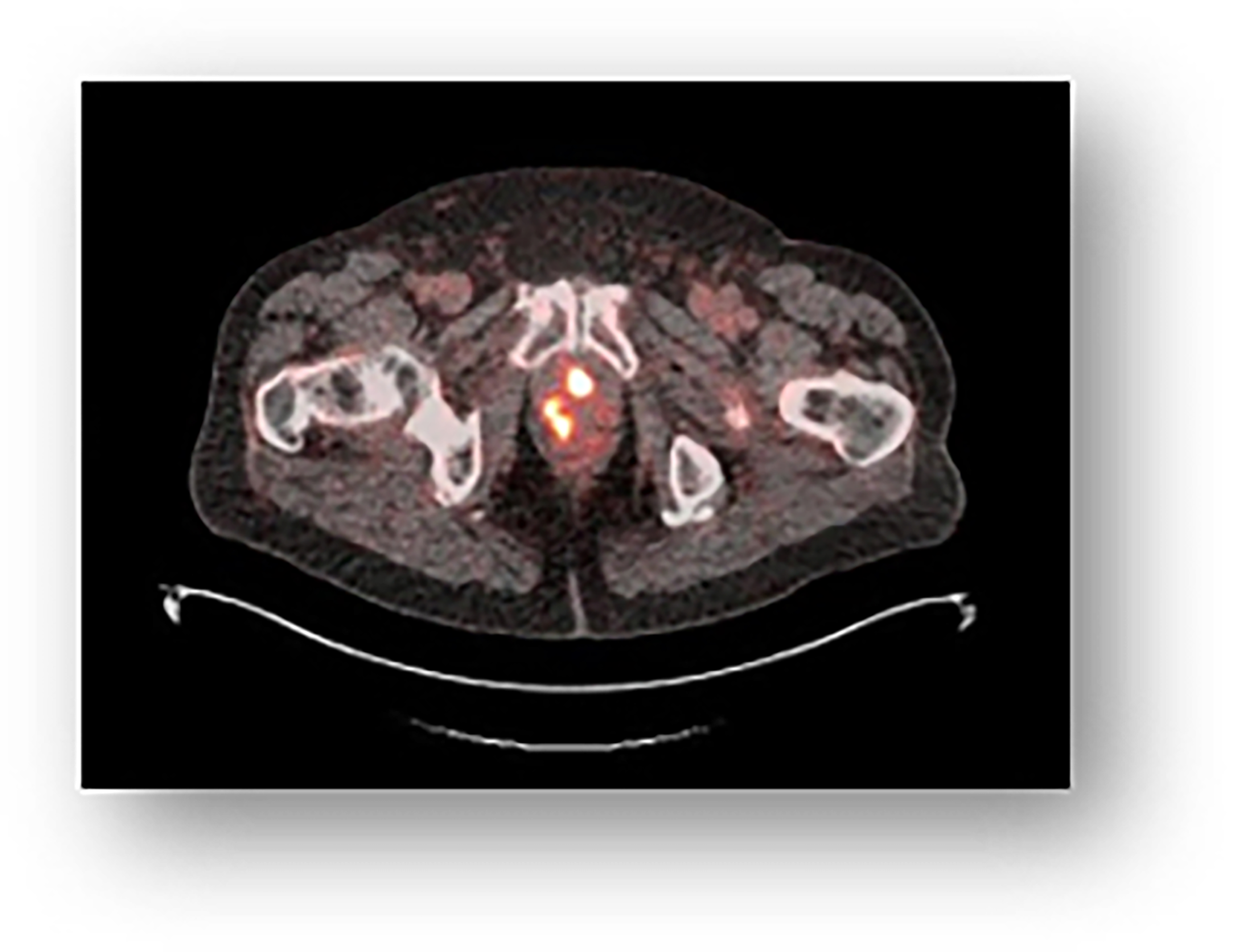
Blue Earth Diagnostics, a Bracco company, announced the signing of a non-exclusive data-sharing agreement with Siemens Healthineers for anonymized POSLUMA (flotufolastat F 18) injection (formerly known as 18F-rhPSMA-7.3) clinical data and images from Blue Earth Diagnostics’ Phase 3 LIGHTHOUSE trial in newly diagnosed prostate cancer. Siemens Healthineers plans to evaluate the data to enhance its analytics and artificial intelligence (AI)-based algorithms for prostate cancer image quantification and interpretation across its advanced PET/CT imaging software. 18F-flotufolastat is an optimized, high-affinity radiohybrid (rh) Prostate-Specific Membrane Antigen-targeted agent approved in the United States for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
“Blue Earth Diagnostics is committed to helping men with prostate cancer across the care continuum, and we recognize the importance of AI in advancing healthcare,” said David Gauden, DPhil, Chief Executive Officer, Blue Earth Diagnostics. “AI-based algorithms have the potential to streamline the PET/CT analytical workflow for hospitals and imaging centers by efficiently providing physicians with information critical to patient management and care. Blue Earth Diagnostics has a long-standing relationship with Siemens Healthineers, a leading medical technology company pioneering breakthroughs in healthcare.”
Dr. Gauden continued, “We are excited to provide these anonymized POSLUMA data from our LIGHTHOUSE trial, for use in enhancing the Siemens Healthineers syngo.via platform. We also plan to make analytical data from the Phase 3 SPOTLIGHT trial of POSLUMA available in the future. POSLUMA represents a new class of high-affinity PSMA-targeted radiopharmaceuticals based on novel radiohybrid technology, and provides physicians with clinically useful information based on its performance at low PSA levels, PSMA binding and low urinary bladder activity. Our product is included in nationally recognized clinical oncology guidelines for prostate cancer, alongside and for all the same categories as the other currently FDA-approved PSMA PET radiopharmaceuticals, and covered by the vast majority of insurance plans. POSLUMA is labeled with the radioisotope fluorine-18 (18F) to leverage high image quality and to enable broad, readily available geographic access for patients via the manufacturing and distribution network of our commercial US manufacturer and distributor, PETNET Solutions Inc, A Siemens Healthineers Company.”
“We believe that the LIGHTHOUSE trial data will be enormously helpful in tailoring our AI technology to support the quantification and clinical interpretation of POSLUMA PET/CT images and are pleased to collaborate with Blue Earth Diagnostics on this data-sharing agreement,” said Bruce Spottiswoode, PhD, Director, Clinical Applications Research, Siemens Healthineers. “The neural networks we are using have been shown to learn radiotracer-specific PET uptake, and we expect them to more efficiently identify clinically relevant features in 18F-flotufolastat images.”
The LIGHTHOUSE clinical trial (NC04186819) was an open-label, prospective, Phase 3, multi-center, single-dose, imaging study investigating the safety and diagnostic performance of POSLUMA PET imaging in men with newly diagnosed unfavorable intermediate-risk, high-risk, or very high-risk prostate cancer. The study enrolled 356 patients at clinical sites in the United States and Europe. The SPOTLIGHT study (NCT04186845) was an open-label, prospective, Phase 3, multi-center, single-dose, imaging study investigating the safety and efficacy of POSLUMA PET imaging in men with suspected prostate cancer recurrence. The study enrolled 391 patients at clinical sites in the United States and Europe.
Related Articles
Citation
Blue Earth Diagnostics, Siemens Healthineers to Collaborate on AI-Based Tools for Prostate PET Imaging. Appl Radiol.
May 8, 2024