POSLUMA PET May Help Guide Prostate Treatment Decisions in Men With Biochemical Recurrence and Low PSA Levels
Images
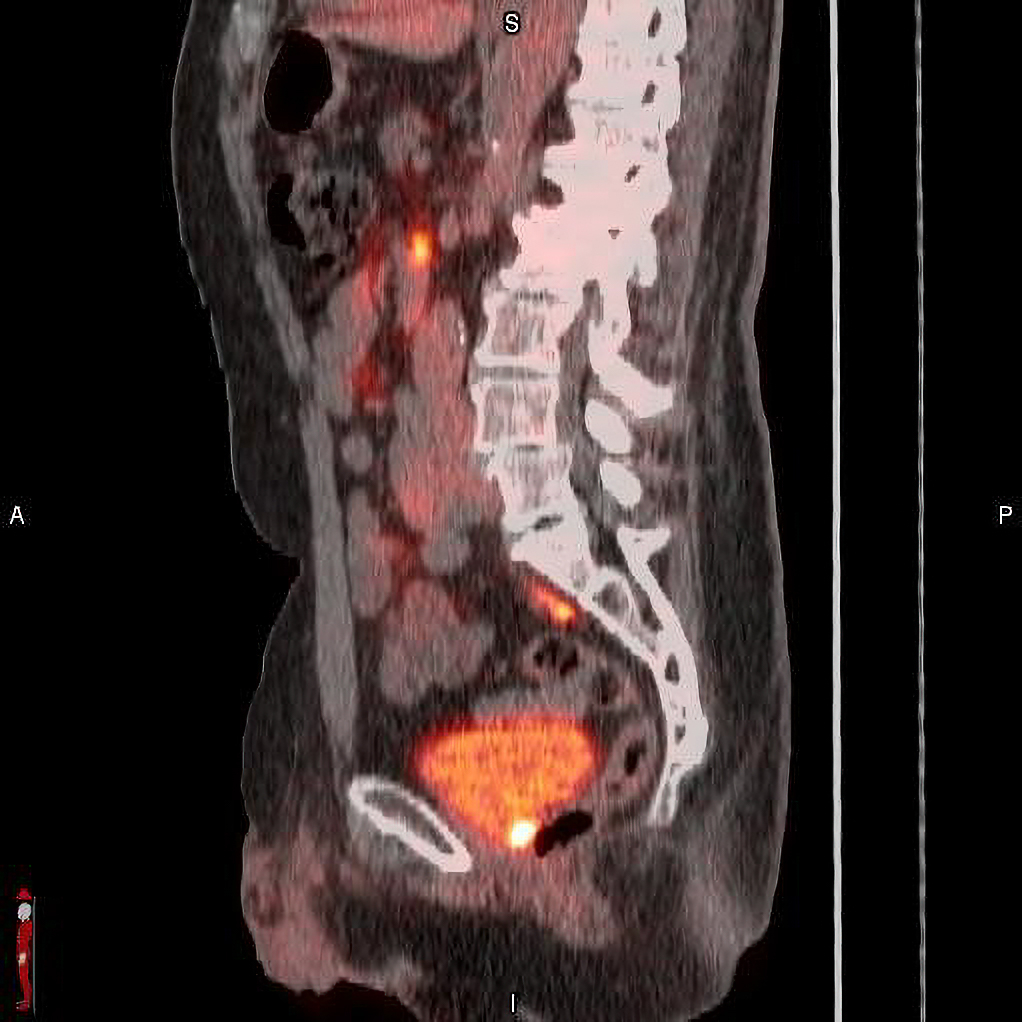
Results from a post-hoc analysis from the Phase 3 SPOTLIGHT trial (NCT04186845) that investigated the use of POSLUMA (flotufolastat F 18) PET in suspected biochemical recurrence of prostate cancer. The analysis examined the detection rate (% positive PET scans) in a subset of patients with low-very low Prostate Specific Antigen (PSA) levels. POSLUMA (flotufolastat F 18) injection (formerly referred to as 18F-rhPSMA-7.3) is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level. The results presented at the American Society for Radiation Oncology (ASTRO) Annual Meeting include:
- Overall, 68% (128/188) of evaluable patients with a PSA level of <1 ng/mL, 76% (51/67) of patients with a PSA of ≥0.5 – <1 ng/mL, and 64% (77/121) of patients with a PSA <0.5 ng/mL had a positive flotufolastat F 18 scan by majority read.
- Extrapelvic lesions were observed in 21% (25/121) of patients with a PSA <0.5 ng/mL, increasing to 39% (26/67) in patients with a PSA of ≥0.5 to 1 ng/mL.
“Recurrent prostate cancer presents clinical challenges, and the ability to determine the extent and location of recurrent disease is necessary to inform physicians and their patients for appropriate clinical management,” said Ashesh B Jani, MD, MSEE, FASTRO, Winship Cancer Institute of Emory University, Atlanta, GA, on behalf of the SPOTLIGHT Study Group. “The SPOTLIGHT study investigated the diagnostic performance of POSLUMA PET imaging as a potential decision-making aid in assessing suspected biochemical recurrence of the disease, and demonstrated precision diagnostic performance, with an overall 83% (322/389) detection rate. This post-hoc analysis further examined POSLUMA performance in 188 men with low-very low PSA levels. Results showed that more than two-thirds of these men were found to have positive POSLUMA scans, with a quarter of them having extrapelvic lesions. POSLUMA PET may be a useful tool for treatment planning, particularly in patients with suspected early recurrence of disease who may be candidates for curative salvage therapy.”
“We are pleased to present these results to the radiation oncology community at ASTRO,” said David E. Gauden, DPhil, Chief Executive Officer of Blue Earth Diagnostics. “POSLUMA has recently been added to nationally recognized clinical oncology guidelines for prostate cancer, alongside and for all the same categories as the other currently FDA-approved PSMA PET radiopharmaceuticals. Our new product represents a new class of high-affinity PSMA-targeted radiopharmaceuticals based on novel radiohybrid technology and provides physicians with high-quality information based on these good detection rates at low PSA levels, high-affinity PSMA binding and low urinary bladder activity. The product is labeled with the radioisotope fluorine-18 (18F) to leverage high image quality and to enable broad, readily available geographic access for patients via the manufacturing and distribution network of our commercial US manufacturer and distributor, PETNET Solutions Inc, A Siemens Healthineers Company.”
The findings were discussed in an oral presentation at the 2023 ASTRO Annual Meeting on October 2, 2023, “Detection Rate of 18F-rhPSMA-7.3 PET in Patients with Suspected Prostate Cancer Recurrence at PSA Levels <1 ng/mL: Data from the Phase 3 SPOTLIGHT Study,” by Ashesh B Jani, MD, MSEE, FASTRO, Winship Cancer Institute of Emory University, Atlanta, GA, on behalf of the SPOTLIGHT Study Group.
The post-hoc analysis of SPOTLIGHT data determined flotufolastat F 18 detection rates (DR) at low-very low PSA levels. Patients enrolled in SPOTLIGHT underwent PET with scans evaluated by majority read of 3 blinded central readers. For the present analysis, all patients with an evaluable flotufolastat F 18 PET and a baseline PSA <1 ng/mL were selected. Overall (patient-level) and regional DR by majority read were determined, stratifying DR according to the patients’ baseline PSA level (<0.2, ≥0.2 – <0.3, ≥0.3 – <0.5, and ≥0.5 – <1 ng/mL).
- In total, 389 patients (median [range] PSA, 1.10 [0.03–135] ng/mL, 84 with intact prostate) had an evaluable flotufolastat F 18 scan. The overall DR was 83% (322/389) by majority read. Of the 389 patients with an evaluable flotufolastat F 18 scan, 188 had a baseline PSA <1 ng/mL and were eligible for the present analysis. Despite low patient numbers in some PSA categories, moderate to high DR were observed, with the patient-level DR shown to increase with increasing baseline PSA. Overall, 68% (128/188) of patients with a PSA <1 ng/mL and 64% (77/121) of patients with a PSA <0.5 ng/mL had a positive flotufolastat F 18 scan by majority read. Regional DRs were broadly consistent across all PSA categories. Extrapelvic lesions were observed in 21% (25/121) of patients with a PSA <0.5 ng/mL, increasing to 39% (26/67) in patients with a PSA of ≥0.5 to 1 ng/mL.
- No serious adverse reactions were attributed to flotufolastat F 18 in the SPOTLIGHT study. Overall, 16 (4.1%) patients had at least one treatment-emergent adverse event that was considered possibly related/related to flotufolastat F 18. The most frequently reported events were: hypertension: 1.8% (n=7); diarrhea: 1.0% (n=4); injection site reaction: 0.5% (n=2), and headache: 0.5% (n=2).
Related Articles
Citation
POSLUMA PET May Help Guide Prostate Treatment Decisions in Men With Biochemical Recurrence and Low PSA Levels . Appl Radiol.
October 3, 2023