Total aganglionosis associated with ileal atresia and malrotation
By Mandell G, Biyyam D, Jorgensen S
Case summary
A 2.2 kg infant girl born via uncomplicated vaginal delivery at 32 weeks’ gestational age with congenital schizencephaly presented with abdominal distention and vomiting.
Imaging Findings
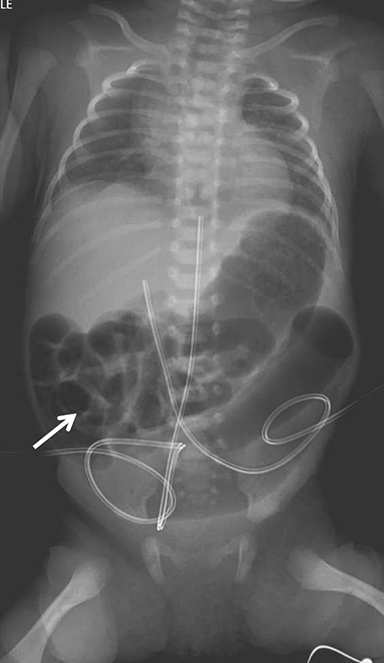
Initial abdominal radiograph showed distended, air-filled loops of bowel. The majority of small bowel was located in the right upper quadrant, suggesting malrotation; however, a focal distended loop of bowel was present in the left lower quadrant (Figure 1).
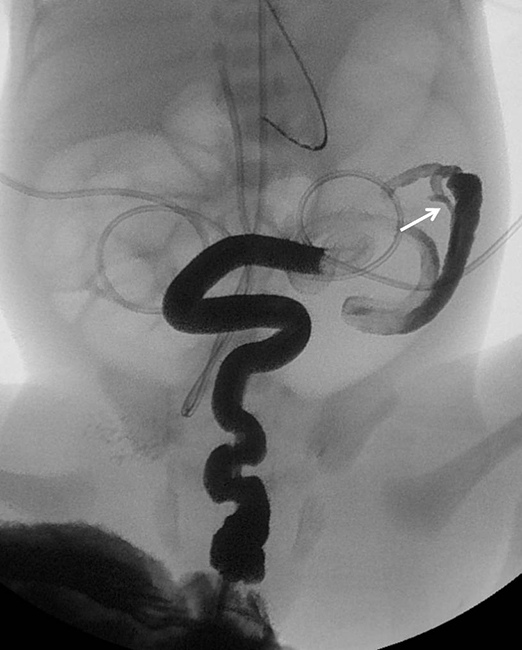
On the second day of life, a contrast enema was performed, demonstrating a microcolon with the cecum and appendix located in the left upper quadrant, confirming malrotation (Figure 2). Contrast could not be refluxed into the distal ileum. A diagnosis of distal ileal atresia was made, resulting in distal ileocecal resection with an ileocolic anastomosis. During surgery no volvulus was noted.
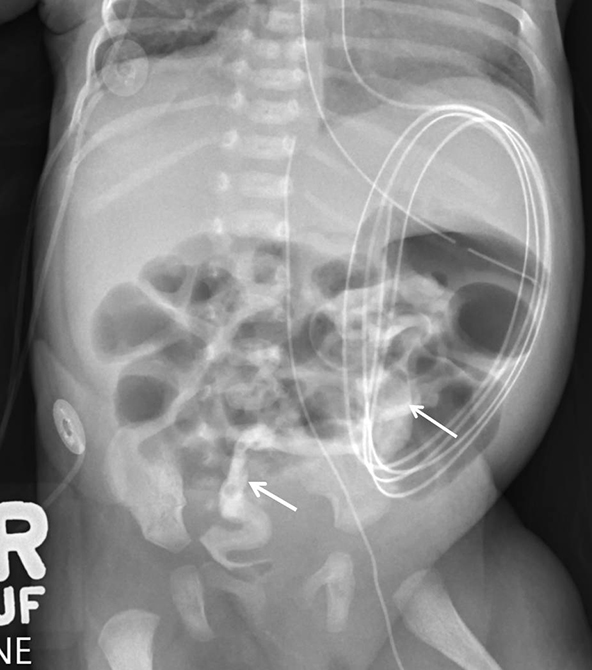
Contrast remained in the microcolon for 15 days. During this time, there was gradual bowel distention, suggesting a recurrent bowel obstruction (Figure 3). With concern for a stricture, a repeat contrast enema was performed on the 22nd day of life. This study demonstrated a microcolon but no stricture or obstruction at the anastomosis.
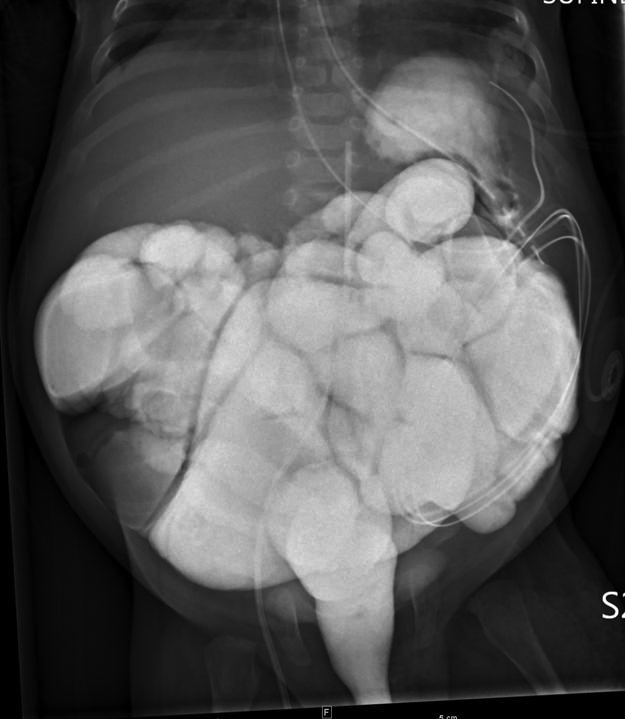
Another contrast enema was performed at 2 months of age because of repeated bowel distention and reduced bowel movements. Initial filling was limited to the colon and distal ileum. The recto-sigmoid ratio was normal. Transition zone was proximal to the microcolon. No anastomotic stricture was detected. Over the next few days retrograde movement of contrast was noted through the entire small bowel to the stomach (Figure 4).
Rectal suction biopsies performed 2 days prior to surgery showed absence of ganglion cells. At definitive surgery, no ganglion cells were seen in the transverse colon. Small bowel with ganglion cells was isolated for enterostomy. In retrospect the resected distal ileum and cecum were analyzed and showed absence of ganglion cells, supporting the diagnosis of total aganglionosis.
Diagnosis
Total aganglionosis associated with ileal atresia and malrotation
Discussion
The incidence of jejunal and ileal atresia ranges from 1 in 1,500 to 1 in 12,000 births.1 Jejunal atresia (JA) is more commonly associated with other congenital anomalies than is ileal atresia (IA). The association of IA and total aganglionosis (TA) is rare, but 21 cases are reported in the literature.2 Malrotation with or without volvulus certainly can be associated with JA and duodenal atresia; however, it is uncommon with isolated IA. The coexistence of IA, TA and malrotation complicated our imaging evaluation of this patient’s distal bowel obstruction. There has been only one other reported case of an infant with combination of ileal atresia, malrotation and total colonic aganglionosis.3
Initial abdominal radiographs demonstrated multiple distended loops of bowel, suggesting distal bowel obstruction. To further evaluate neonates with a distal bowel obstruction, a contrast enema should be performed. Microcolon is a nonspecific finding appreciated in meconium ileus, IA and TA. While TA is rare, findings of a short rigid colon with loss of redundancy and retention of contrast in the colon for many days suggested the diagnosis. Recurrence of bowel distention following ileocolostomy for repair of IA should always raise suspicion for another more distal obstructive problem. In our patient an anastomotic stricture was contemplated. Repeat contrast enemas demonstrated no stricture or stenotic anastomosis but did demonstrate retrograde peristalsis through the small bowel to the stomach, another sign of TA. Hirschsprung’s disease (HD) or aganglionosis is the premature arrest of the craniocaudal migration of vagal neural crest cells in the hindgut between the 5th and 12th week of gestation to form the enteric nervous system and is regarded as a neurocristopathy.4 The incidence of HD is estimated at 1 in 5000 live births. There is a preponderance of affected males compared to females (4:1). Patients are classified as having short-segment HD when the aganglionic segment does not extend beyond the upper sigmoid, and long segment HD when the aganglionic segment extends proximal to the sigmoid colon.5 TA is rare (3–8% of cases of HD). Congenital anomalies occur in 18% of patients with HD and include malrotation, colonic atresia and imperforate anus.5
The midgut is the portion of the intestines supplied by the superior mesenteric artery, extending from the duodenum to the mid-transverse colon. An ischemic event is considered the most likely cause of jejunoileal atresia and is thought to occur between the 6th and 8th weeks of gestation. In a series of 114 infants with jejunoileal atresia, the level of the atresia was the jejunum in 62%, the ileum in 30%, and both the jejunum and ileum in 8%.6 Associated extra-intestinal anomalies occur more frequently in patients with JA (42%) than in patients with IA (2%), suggesting that the former probably occurs earlier in utero than the latter.7 The combination of IA and TA is extremely rare. One possible mechanism for this occurrence is a vascular accident that results in atresia and acts as a barrier to caudal migration of the neurenteric cells distal to the atresia.
Malrotation is associated with jejunoileal atresia in 17% of patients. Approximately 50% of these patients have midgut volvulus at the time of diagnosis. A prior study showed that malrotation is associated with other congenital anomalies in 62% of patients.8 In this series of 34 patients there were four cases of HD. In total, 21% of patients with malrotation and an associated anomaly had jejunal atresia.8 There were no cases of ileal atresia in this series.
There has been only one other reported case of IA, TA, and malrotation.3 As in our patient, no volvulus was present as etiology of the atresia.
Conclusion
Both IA and TA can present with distal bowel obstruction in the neonate and microcolon on contrast enema. While this case is rare, it is important for the radiologist to know that malrotation, bowel atresias and HD can all be associated with other congenital anomalies.
References
- Best K, Tennant P, Addor M, et al. Epidemiology of small intestinal atresia in Europe: A register-based study. Arch Dis Child Fetal Neonatal Ed. 2012; 97:F353.
- Daher P, Raffoul L, Riachy E, et al. Uncommon co-occurrence of ileal atresia and total colonic aganglionosis in two unrelated newborns. Pediatr Surg Int. 2012; 28:85-87.
- Gordon G, Wisbach W, Vazquez D. Ileal atresia, malrotation and hirschsprung’s disease. A case report. Ped Surg Case Reports. 2013; 1: e3ee5.
- Moore S, Rode H, Millar A, et al. Intestinal atresia and hirschsprung’s disease. Ped Surg Int. 1990; 5:182-184.
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008; 45: 1-14.
- Stollman T, deBlaauw I, Wijnen M, et al. Decreased mortality but increased morbidity in neonates with jejunoileal atresia; a study of 114 cases over a 34-year period. 2009; J Ped Surg. 44:217-221.
- Sweeney B, Rajendra S, Prem S. Jejunoileal atresia and associated malformations: Correlation with timing of in utero insult. J Ped Surg. 2001;36:774-778.
- Filston H, Kirks D. Malrotation - the ubiquitous anomaly. J Ped Surg. 1981;16:614-620.
