SyMRI 3D Nets CE Marking for 2024 Commercial Launch
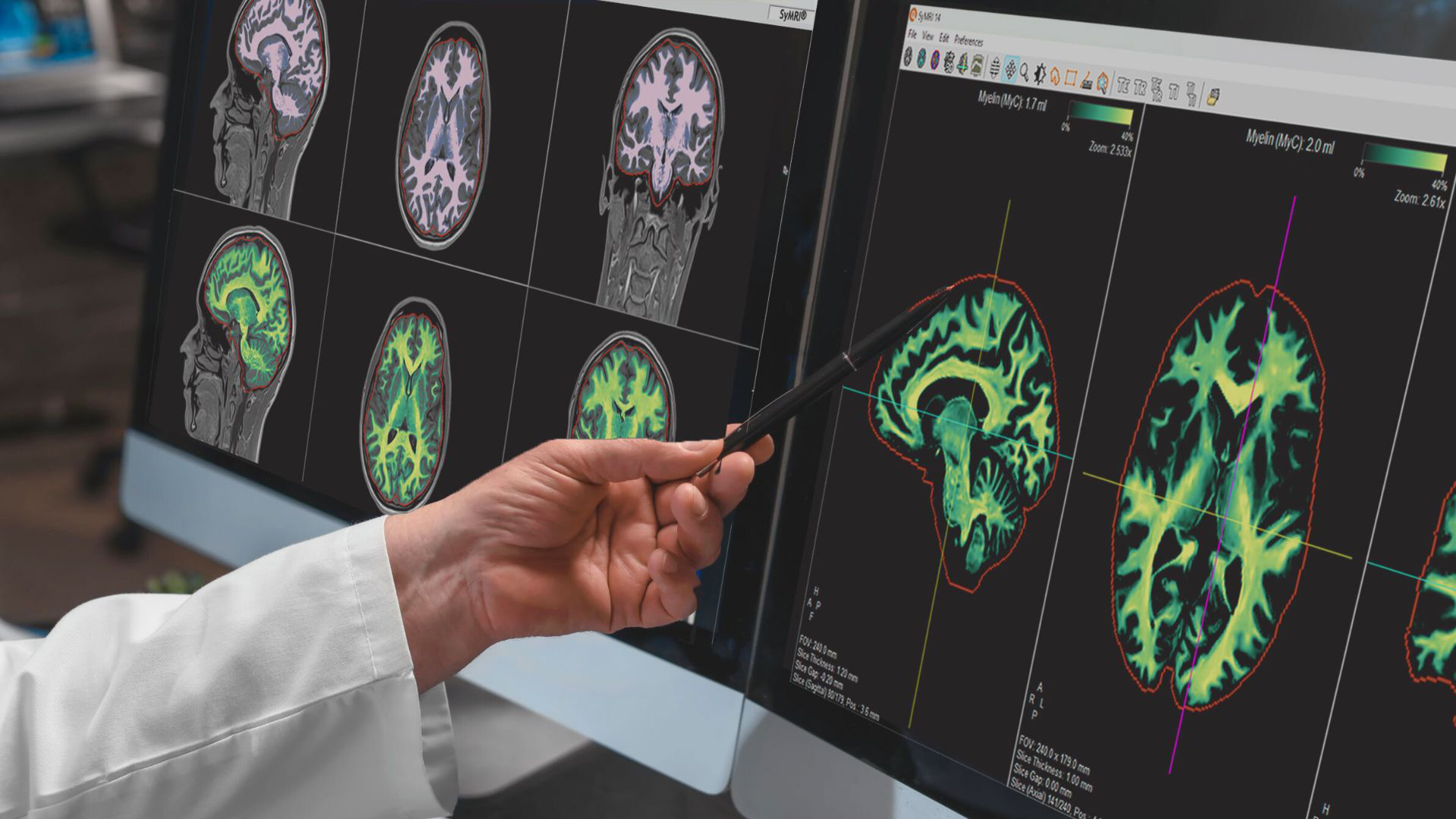
SyntheticMR has received CE Marking for its SyMRI 3D imaging solution with isotropic 3D resolution, readying the solution for a 2024 commercial launch.
"We are delighted to see our 3D solution CE-marked and available on the European market,” says Ulrik Harrysson, CEO at SyntheticMR AB. “SyMRI 3D represents the next generation of quantitative MRI, offering unprecedented resolution and accuracy in brain imaging, revolutionizing the landscape of medical diagnostics."
SyntheticMR’s new 3D release is a next-generation imaging solution that is poised to revolutionize the field of medical imaging by introducing a myriad of innovative applications within SyMRI's post- processing solution. This advanced SyMRI sequence introduces 3D resolution to quantitative values and tissue segmentations, ensuring the same level of precision in quantification as achieved with SyMRI's 2D imaging.
SyMRI 3D enables precise volumetric estimations of brain regions, a technique commonly referred to as parcellation, which empowers clinicians to gain deeper insights into brain structure and function. Furthermore, the exceptional resolution provided by SyMRI 3D facilitates comprehensive lesion analysis, ensuring a more accurate and in-depth assessment of medical conditions.
“This advanced sequence extends 3D resolution to quantitative values and tissue segmentations, matching the precision achieved in SyMRI's 2D imaging. The CE-marking for SyMRI 3D paves the way for physicians to make more informed decisions through quantitative imaging.” says Kyle Frye, CCO & President of SyntheticMR Inc. “Receiving approval for SyMRI 3D will allow us to enable physicians to make better, more informed decisions using quantitative imaging.”
