POSLUMA May Help Identify Recurrence of Prostate Cancer After Radical Prostatectomy
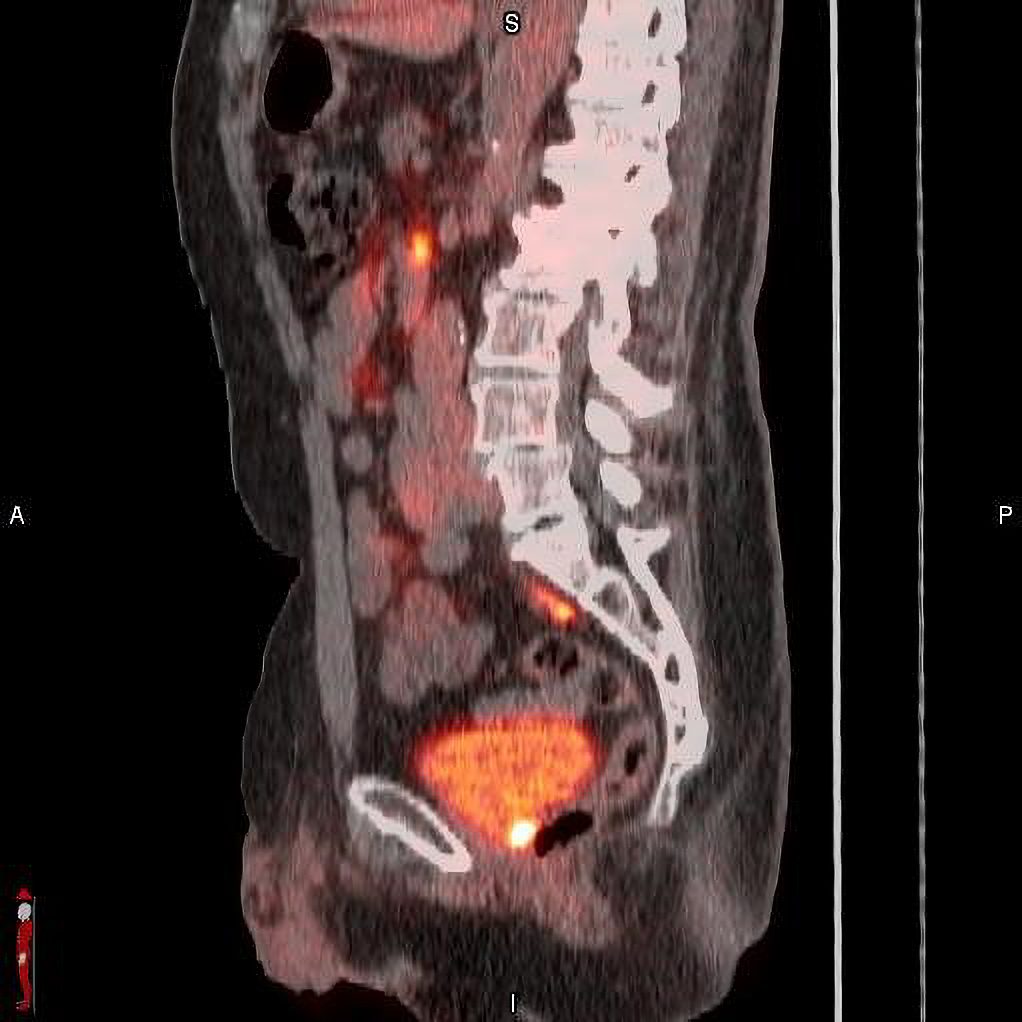
Blue Earth Diagnostics announced results from a post-hoc analysis from the Phase 3 SPOTLIGHT trial (NCT04186845) that investigated the use of POSLUMA (flotufolastat F 18) PET injection in suspected biochemical recurrence of prostate cancer. The findings were discussed in a moderated poster presentation at the 2024 ASTRO Annual Meeting on “18F-Flotufolastat Detection Rates in the Pelvis Region for Patients with Prostate Cancer Recurrence after Radical Prostatectomy and PSA Levels <1 ng/mL: Data from the Phase 3 SPOTLIGHT Study,” by Bridget F. Koontz, MD, FASTRO, AdventHealth Cancer Institute, Orlando, FL for the SPOTLIGHT Study Group. Dr Koontz was affiliated with Duke University Medical Center, Durham, NC, at the time of the study.
The analysis examined the detection rate (% positive PET scans) in the pelvis region for SPOTLIGHT patients who had received radical prostatectomy (RP) only (168/389) and with low-very low Prostate Specific Antigen (PSA) levels of < 1 ng/mL (119/168). Results demonstrated 69% and 67% detection rates for POSLUMA in men with PSA < 1 ng/mL (82/119) and < 0.5 ng/mL (57/85), respectively.
FDA-approved POSLUMA (flotufolastat F 18) is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
“Physicians need actionable information from PSMA PET scans to guide informed clinical management decisions for men with prostate cancer,” said Bridget F. Koontz, MD, FASTRO. “This is of particular importance in the pelvis region, the most common site of recurrence. This analysis explored the performance of POSLUMA in the pelvis region for post-prostatectomy men who were likely in the early stages of disease recurrence. Approximately one-third of patients had detectable recurrence in the prostate bed, suggesting that POSLUMA may be useful in identifying recurrence next to the bladder, and help to guide curative salvage therapy.”
“Findings presented at ASTRO about POSLUMA detection rates in the pelvis region for men with recurrent prostate cancer reinforce important clinical considerations in selecting a precision diagnostic imaging agent for PSMA PET procedures,” said Marco Campione, Chief Executive Officer of Blue Earth Diagnostics. “Activity in the urinary bladder and ureters is a common feature of PSMA PET radiopharmaceuticals, and can potentially obscure tumors and interfere with accurate image interpretation. A post-hoc analysis of POSLUMA clinical trials demonstrated that urinary activity did not impact image assessment for 96% of patients (682/712) by majority read.”
