Photoacoustic Computed Tomography Technology Images Flowing Blood
Researchers at the California Institute of Technology (Caltech) and the University of Southern California have a developed a low-cost, non-invasive 3D imaging method, called Photoacoustic Computed Tomography through an Ergodic Relay (PACTER), capable of imaging flowing blood in real time. In a study in Nature Biomedical Engineering, the authors successfully demonstrated the technique’s feasibility in both animals and humans.
“This technology takes several innovative concepts and packages them into one compact unit. I could see this type of tool potentially finding broad applications, including continuous monitoring in hospitals and at home,” said Randy King, PhD, a program director in the Division of Applied Science & Technology at the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
Factors such as blood-flow velocity and oxygenation can be telling of the early development and status of atherosclerosis, aneurysms, thrombosis, and other conditions. Some clinical imaging techniques can detect these hemodynamic properties, but they involve injecting patients with dyes, called contrast agents, or beaming them with ionizing radiation, both of which can pose health risks.
Work in the Wang lab has recently focused on alternatives based on the photoacoustic effect, a phenomenon that describes the transmission of sound waves following the absorption of light. Photoacoustic imaging leverages this effect to image tissues inside the body. With this technique, biomolecules absorb light from laser pulses, re-emitting their energy as ultrasonic waves. Those reflected signals can then be used to create images.
Wang and his colleagues used photoacoustic imaging to capture signals from the oxygen-carrying molecule hemoglobin, housed in red blood cells, allowing them to visualize flowing blood in real time.
Photoacoustic technology can require a multitude of costly ultrasound sensors to capture rapidly propagating soundwaves. A prior iteration of the team’s system showed promise despite using just one sensor. Still, it could only capture enough information to produce 2D images.
To produce 3D images while keeping the small and cost-effective one-sensor design, the researchers devised a strategy to do much of the heavy lifting before any imaging would take place.
In the new study, the authors developed PACTER, a desktop system that they calibrated using narrow laser beams directed at 6,400 discrete points in a bovine blood sample. While intensive, this calibration step enabled the system to later distinguish 6,400 signals from a single, broader laser beam during imaging experiments. This approach meant the researchers could perform imaging quickly while still capturing abundant data.
“We’re able to decompose the total raw signal into individual signals. In effect, we’re able to just use that single detector as thousands of virtual detectors,” said senior author Lihong Wang, PhD, a professor of medical and electrical engineering at Caltech.
The authors tested PACTER by pulsing laser beams into mice. Sensing the reflected waves, the system successfully mapped out the animals' abdominal vasculature in 3D. It also detected periodic changes in vessel size, allowing the researchers to identify the animals’ breathing rates.
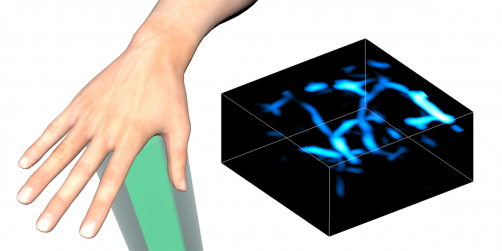
Next, the researchers visualized vessels within the hands and feet of human subjects, locations commonly assessed in patients with peripheral vascular disease and diabetes.
With their measurements, the team was able to calculate blood flow speeds. By applying blood pressure monitor cuffs, the researchers could alter the hemodynamics within the subjects’ appendages. The authors successfully spotted expected changes in the velocity of blood and in the shape of vessels.
“The fact that we managed to fit so much capability into a cost-effective system is a quantum leap forward for us. But there’s still more work to do. This is not the final version of this technology,” Wang said.
The researchers seek to improve PACTER’s sensitivity to signals in the body and scale it down into a more portable size. As for applications, they have their sights set on using it to measure the oxygenation of blood in arteries and veins in the neck and to track metabolism in the brain. But this is just one of many possible avenues to proceed down.
As a single group, the team can only explore so much, Wang explained. He hopes that the study inspires others to tackle the numerous clinical challenges he already has in mind and to unveil new potential uses.
