Delineating extent of disease using contrast-enhanced digital mammography
By Patel BK

CASE SUMMARY
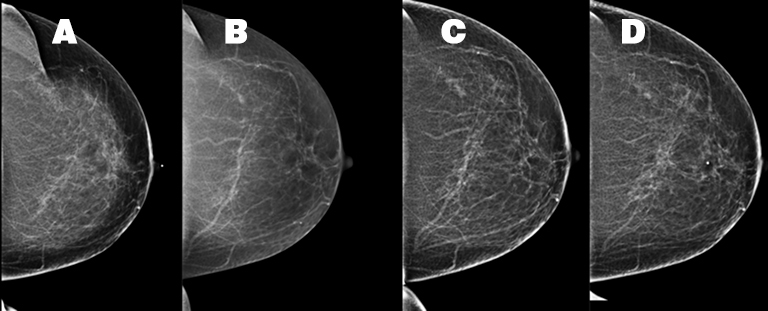
A 54-year-old Caucasian woman presented with a painful left breast lump. The patient’s medical history was significant and included a pituitary adenoma, a hysterectomy, and a unilateral oophorectomy at age 53. The patient’s menopausal status was unknown but she had no history of hormone replacement therapy. The patient’s family history included her mother’s diagnosis of melanoma and inflammatory breast cancer at age 60. Prior mammograms were conducted in 2009, 2012 and 2014 (Figure 1). A physical examination of the left breast demonstrated an area of firmness within the superior left breast and a small, mobile, palpable left axillary mass, assumed to be a lymph node. There were no overlying inflammatory changes.
IMAGE FINDINGS
Tomosynthesis
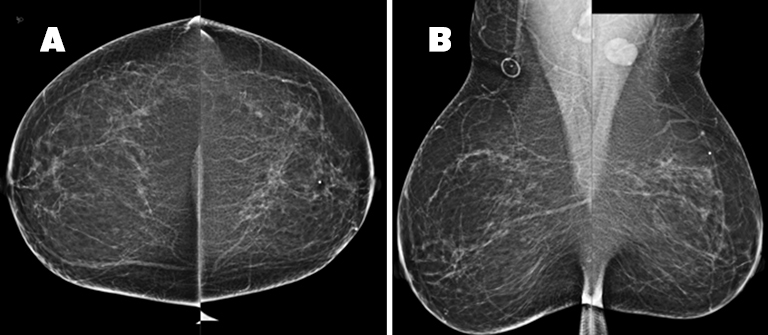
The patient received a bilateral 3D mammogram, which demonstrated scattered fibroglandular densities in both breasts. Corresponding to the BB placed over the left upper, central breast area of palpable concern, there is an ill-defined area of architectural distortion in the upper left breast, best seen on tomosynthesis imaging. The left axillary lymph node is enlarged, measuring 2.6 cm (Figure 2). No suspicious microcalcifications are seen. There is no evidence of malignancy in the right breast.
Breast ultrasound
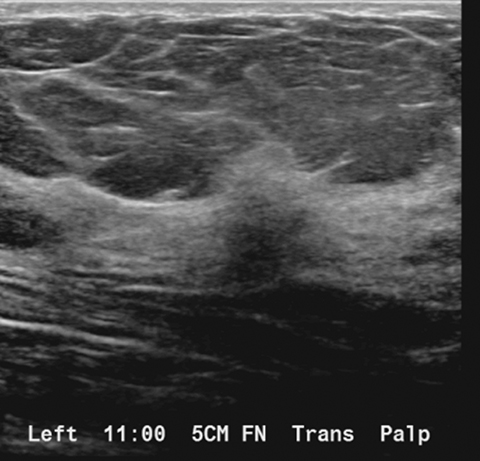
A targeted left breast ultrasound at 11 o’clock, 5 cm from the nipple, underlying the area of a palpable abnormality, demonstrates an ill-defined hypoechoic mass with posterior acoustic shadowing measuring 1.2 × 1.3 × 0.7 cm (Figure 3). It is difficult to determine if this subtle ultrasound finding corresponds with the tomosynthesis finding of an ill-defined area of architectural distortion in the upper left breast. A subsequent targeted left axillary ultrasound demonstrated a 2.0 × 1.5 × 1.3 cm enlarged lymph node. The greatest cortical thickness measured 7 mm.
The patient subsequently received a contrast-enhanced digital mammogram (CEDM) because it was unclear if the ultrasound finding corresponded with the initial architectural distortion observed on tomosynthesis. CEDM was also used to better evaluate the extent of abnormal findings.
Contrast-enhanced digital mammography
There is minimal symmetric background parenchymal enhancement.
Subtracted images
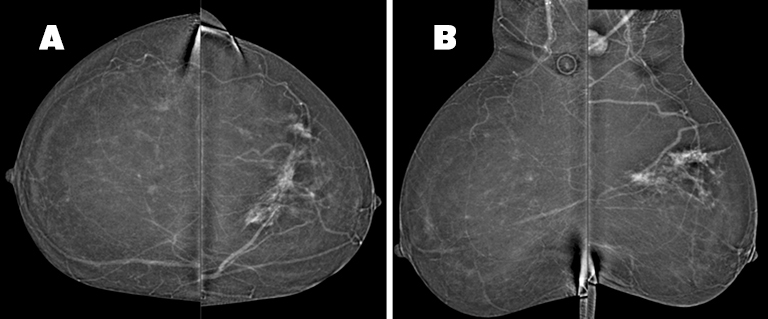
In the upper left breast, middle depth, there is a 6.5(AP) × 8.4(ML) ×4.0(CC) cm area of non-mass enhancement, a portion of which corresponds to the area of architectural distortion seen on tomosynthesis. The non-mass enhancement involves both the medial and lateral portions of the upper left breast and is suspicious for multi-centric disease (Figure 4). An enlarged, enhancing left axillary lymph node is discernable. There is no suspicious enhancing mass or non-mass enhancement observed in the right breast.
The patient received a tomosynthesis-guided biopsy of the architectural distortion in the left breast and ultrasound-guided biopsy of the suspicious left axillary lymph node.
DIAGNOSIS
- Architectural distortion with associated non-mass enhancement within the upper left breast was consistent with infiltrating ductal carcinoma, estrogen receptor positive, progesterone receptor positive, and HER-2/neu negative.
- Enlarged left axillary lymph node was consistent with metastatic lymph node involvement.
DISCUSSION
Contrast-enhanced digital mammography, a commercially available breast imaging modality, is a low-cost imaging tool that provides both a low-energy image comparable to that of digital mammography and a contrast-enhanced reconstructed image similar to that of MRI. Contrast enhancement highlights the neoangiogenesis of breast tumors and is the underlying principle leveraged by both breast MRI and breast CT.4 Studies have demonstrated that CEDM improves diagnostic accuracy in comparison to both mammography and ultrasound as it further enhances lesion conspicuity.1
The lower reported sensitivity of mammography in women with dense breasts is most likely related to a masking effect caused by the large amount of fibroglandular tissue. This particular case, however, demonstrates the ability of CEDM to reveal the extent of disease largely underestimated by both ultrasound and standard mammography despite this patient’s non-dense breast tissue.
CEDM was initially recommended to better delineate the abnormality prior to biopsy. Although ultrasound showed a mass of only 1.2 cm, CEDM demonstrated the extent of abnormal enhancement to be greater, measuring 8.5 cm in superior-inferior extent. As a result, the CEDM findings altered clinical management, with the patient receiving neoadjuvant chemotherapy followed by breast conservation therapy. Postsurgical pathology demonstrated residual carcinoma measuring 3.5 cm in a treated tumor bed measuring up to 7 cm (Figure 5).
Studies comparing CEDM to MRI determined that the modalities exhibit similar sensitivity with regard to breast cancer detection 2, 3 but higher specificity with CEDM in regard to identifying additional foci in the ipsilateral breast.3 This case illustrates CEDM’s ability to indicate tumor size and extent, which could play a potential role in both pre-surgical and treatment planning.
While MRI is currently the standard of care for contrast-enhanced breast imaging, CEDM may be an alternative with comparable cancer detection sensitivity. This case also demonstrates that CEDM may be considered as an alternative preoperative measurement tool in breast cancer patients and those with limited access to or contraindications to MRI.
CONCLUSION
Diagnosing the extent of disease is an important factor in treatment planning for breast cancer patients. Studies have demonstrated that CEDM may be an effective alternative to MRI, as they achieve comparable sensitivity. CEDM also provides the advantage of lower cost and shorter procedure time compared to breast MRI, and may be considered a viable option for patients requiring additional imaging to determine the extent of their disease for pre-surgical and treatment planning.
REFERENCES
- Dromain C, Thibault F, Diekmann F, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results of a multireader, multicase study. Breast Cancer Res. 2012 Jun 14;14(3):R94.
- Fallenberg EM, Dromain C, Diekmann F, et al. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Diagn Interv Imaging. 2014 Mar;95(3):351-2.
- Jochelson MS, Dershaw DD, Sung JS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology. 2013:266(3):743-51.
- Lewis TC, Pizzitola VJ, Giurescu ME, Eversman WG, Lorans R, Robinson KA, Patel BK.Contrast-enhanced Digital Mammography: A Single-Institution Experience of the First 208 Cases. Breast J. 2016 Oct 2. doi: 10.1111/tbj.12681. [Epub ahead of print]
