Clinical Applications of Gadopiclenol in Breast MRI
By Basak Dogan, MD, FSBI
SPONSORED BY BRACCO
Introduction
Breast cancer is the second most common cancer diagnosed in women, affecting millions globally.1 The higher sensitivity of MRI for breast cancer detection depends on gadolinium-based contrast agents (GBCAs).2-4 With more than 700 million doses administered worldwide, GBCAs are considered safe; however, the risk of serious conditions like nephrogenic systemic fibrosis (NSF) warrants their prudent use.5 Furthermore, mounting evidence on the long-term, albeit trace amounts of gadolinium retention in human tissue, has led to more thoughtful practices around GBCA use and has generated interest in the development of low-dose agents, the most recent of which is gadopiclenol.6-8
In breast MRI, minimizing gadolinium exposure is particularly important, as many healthy women at elevated risk of breast cancer undergo repeat screening MRI with GBCAs, increasing the cumulative dose of gadolinium during their lifetime.9 This article discusses considerations for GBCA use in breast MRI, with a focus on gadopiclenol.
Applications of Contrast-Enhanced Breast MRI
Breast MRI has the highest sensitivity for breast cancer detection among currently available imaging modalities. Since effective mam- mographic assessment can be difficult in women with dense breast tissue or breast implants, contrast-enhanced breast MRI is often preferred for identifying tumors.3,10 Breast MRI is also recommended as a supplemental screening modality for women with an elevated lifetime risk of breast cancer.10 Owing to its exceptional sensitivity, contrast-enhanced breast MRI can aid in the surveillance of breast can- cer survivors with mammographically dense breasts or those diagnosed before age 50, and in assessing tumor response to neoadjuvant chemotherapy to help guide treatment decisions and surgical planning.10
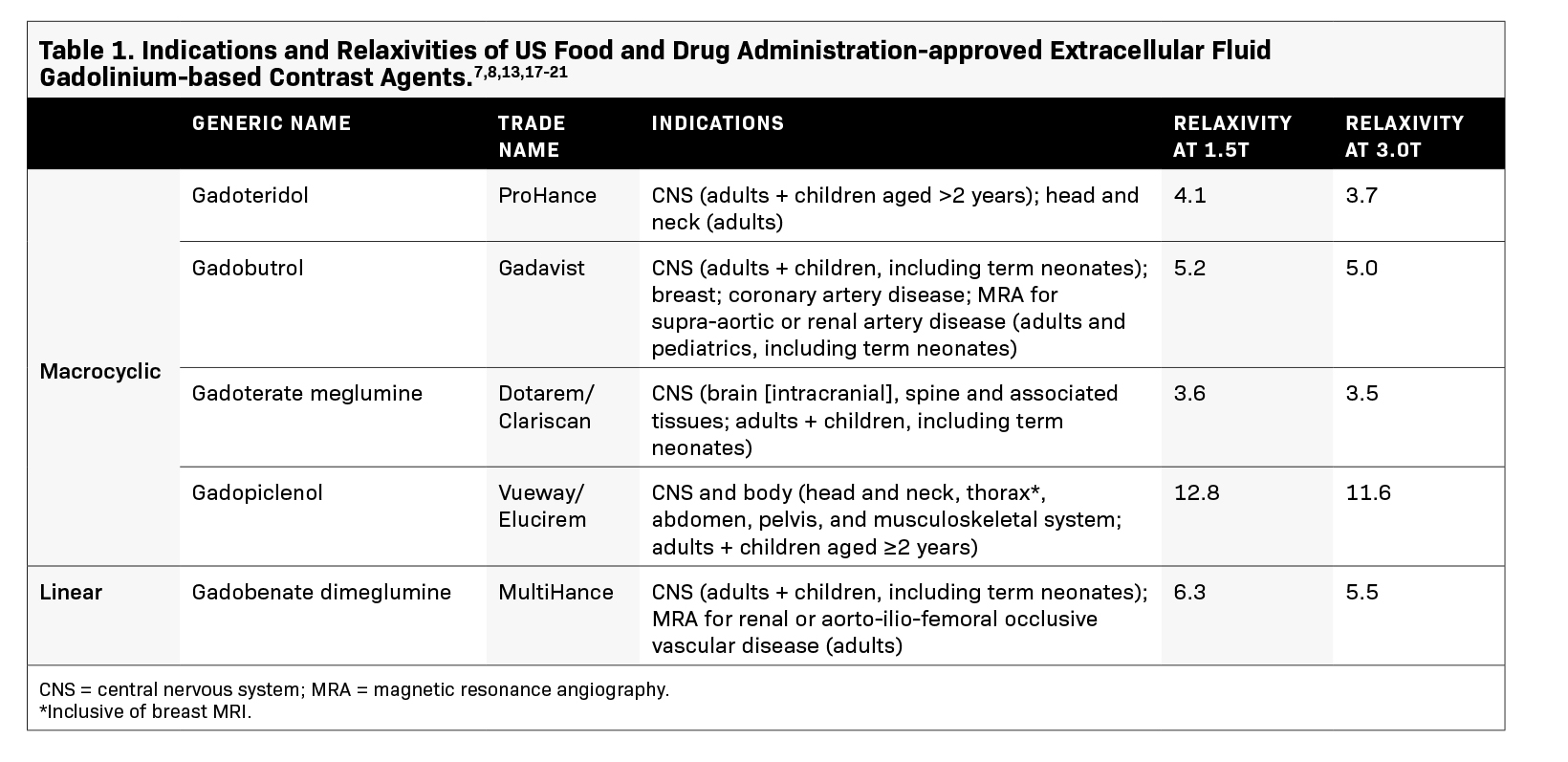
The utility of GBCAs in imaging relies on the paramagnetic properties of gadolinium (Gd3+ ).11 Gadolinium shortens the T1 relaxation time of nearby protons, resulting in increased signal intensity on T1-weighted images.11 This higher signal, which quantitatively differs across GBCAs based on their relaxivity, facilitates the detection and characterization of breast lesions by increasing the image conspicuity of areas with increased vascularity, such as tumors, while suppressing normal breast tissue.12 Currently, 2 GBCAs are approved by the US Food and Drug Administration (FDA) for use in breast MRI (Table 1), with gadopiclenol being the most recent addition and exhibiting the highest relax- ivity.13 In breast imaging, the use of a high relaxivity GBCA that is administered at half the dose of standard extracellular fluid (ECF) agents can allow for lower gadolinium exposure while ensuring an efficacious exam.
Considerations for GBCA Selection: Gadopiclenol for Breast MRI
Weighing the benefits of a GBCA against its risks is critical in ensuring patient safety and appropriateness of contrast-enhanced breast MRI studies.14 Three key factors can help guide contrast selection — efficacy, stability, and safety. All currently available GBCAs differ in terms of efficacy and indications for use, and the choice of an agent in favor of others is often based on its safety profile in addition to its ability to improve lesion conspicuity relative to background and produce high image quality. GBCAs with high relaxiv- ity can increase image enhancement and potentially improve lesion detection, which has been proven in imaging the central nervous system.11 Gadopiclenol exhibits approximately 2 to 3 times higher relaxivity than other approved ECF linear and macrocyclic GBCAs in water and serum and at all field strengths.13
Chelate structure and ionicity influence overall GBCA stability and are thus important considerations in selecting an agent. In general, linear GBCAs have lower stability than macrocyclic agents, with nonionic linear agents being the least stable and linear ionic agents being moderately stable.15 The higher stability of macrocyclic agents is credited to their cage-like structure that binds Gd3+ more tightly than linear chelates.15 In the setting of renal impairment, dissociation of the Gd3+ ion from the GBCA chelate has been linked to the development of NSF.16 In response, the American College of Radiology (ACR) has classified GBCAs relative to the num- ber of NSF cases associated with each agent.14 According to the groupings, gadopiclenol is classified as a Group II agent for which the risk of NSF among patients exposed to standard or lower than standard doses is sufficiently low or possibly nonexistent such that assessment of renal function with a questionnaire or laboratory testing is optional prior to intravenous administration.14
Lastly, ensuring patient comfort during an MRI exam is critical to achieving an adequate study, especially in breast imaging given the prone positioning of patients and the length of the exam if an abbreviated protocol is not used. In terms of GBCA use, those associated with lower rates of immediate adverse reactions, such as nausea, injection site, or allergic reactions, are preferred. The safety profile of gadopiclenol resembles that of other available GBCAs, with most adverse reactions being mild and self-limited.7,8
In addition, the high relaxivity of gadopiclenol ensures lower gadolinium exposure, with the use of an approved dose that is half that of other ECF agents (ie, 0.05 mmol/kg for gadopiclenol vs 0.1 mmol/kg for other ECF agents approved in the US for similar indications).7,8,17-21 This aligns with the ACR’s guidance to administer a GBCA only if deemed necessary and at the lowest dose needed for diagnosis.14
In terms of clinical experience, gadopiclenol 0.05 mmol/kg has been compared with 0.1 mmol/kg in the randomized, double-blind, crossover, phase 3 PROMISE study that enrolled 273 adults with at least 1 suspected focal lesion in 1 of 3 body regions (head and neck; breast, thorax, abdomen, or pelvis; or musculoskeletal system).22 Among the study population were 76 patients with suspected breast lesions. Despite the half dose of gadolinium administered, gadopiclenol was comparable to gadobutrol for overall lesion evaluation, signifying that a GBCA with high relaxivity can reduce gadolinium exposure while maintaining image quality.22 In terms of safety, 12 of 288 (4.2%) participants receiving gadopiclenol and 16 of 290 (5.5%) receiving gadobutrol experienced adverse events related to contrast; however, they did not differ in frequency, intensity, or type.22
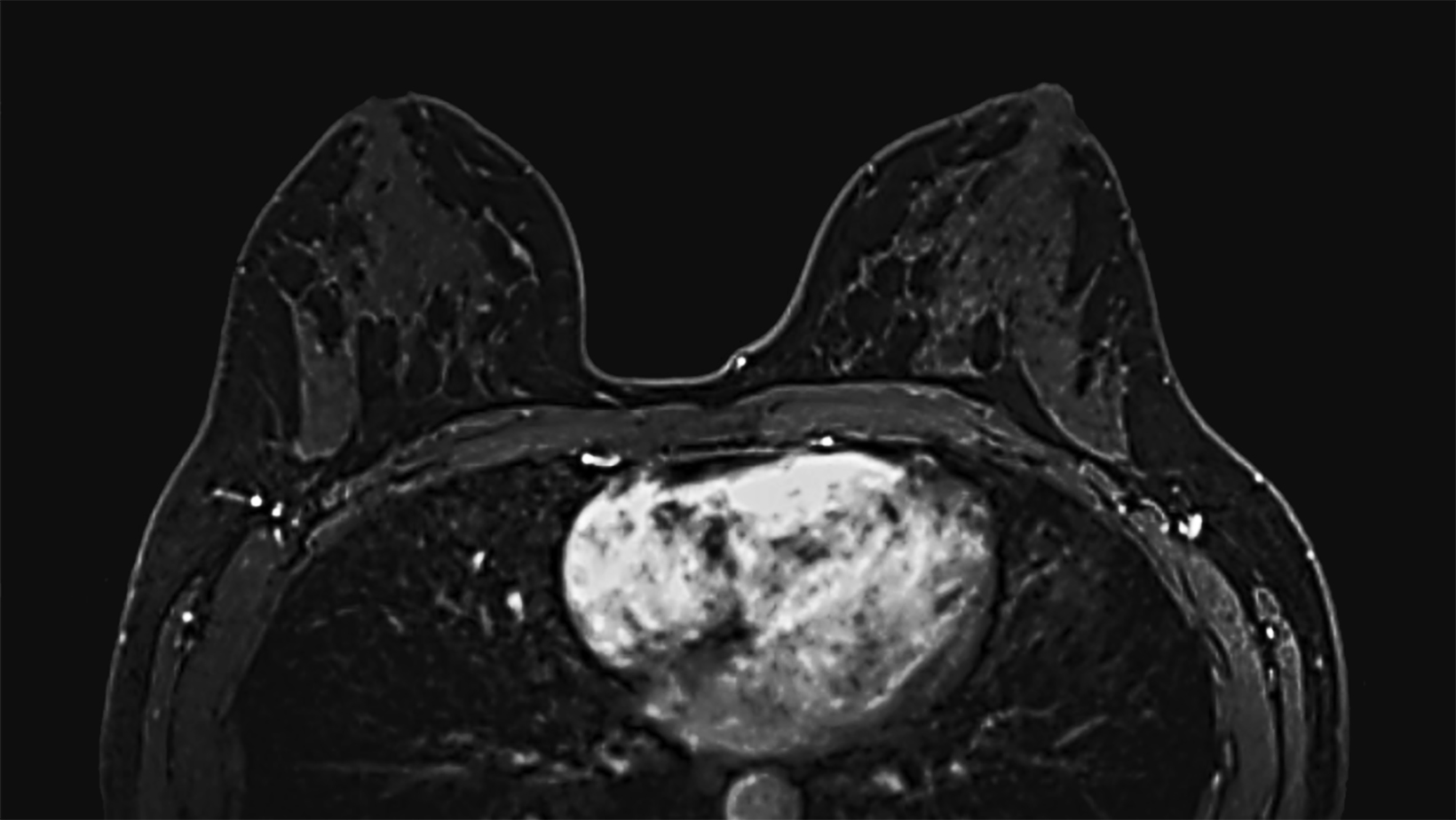
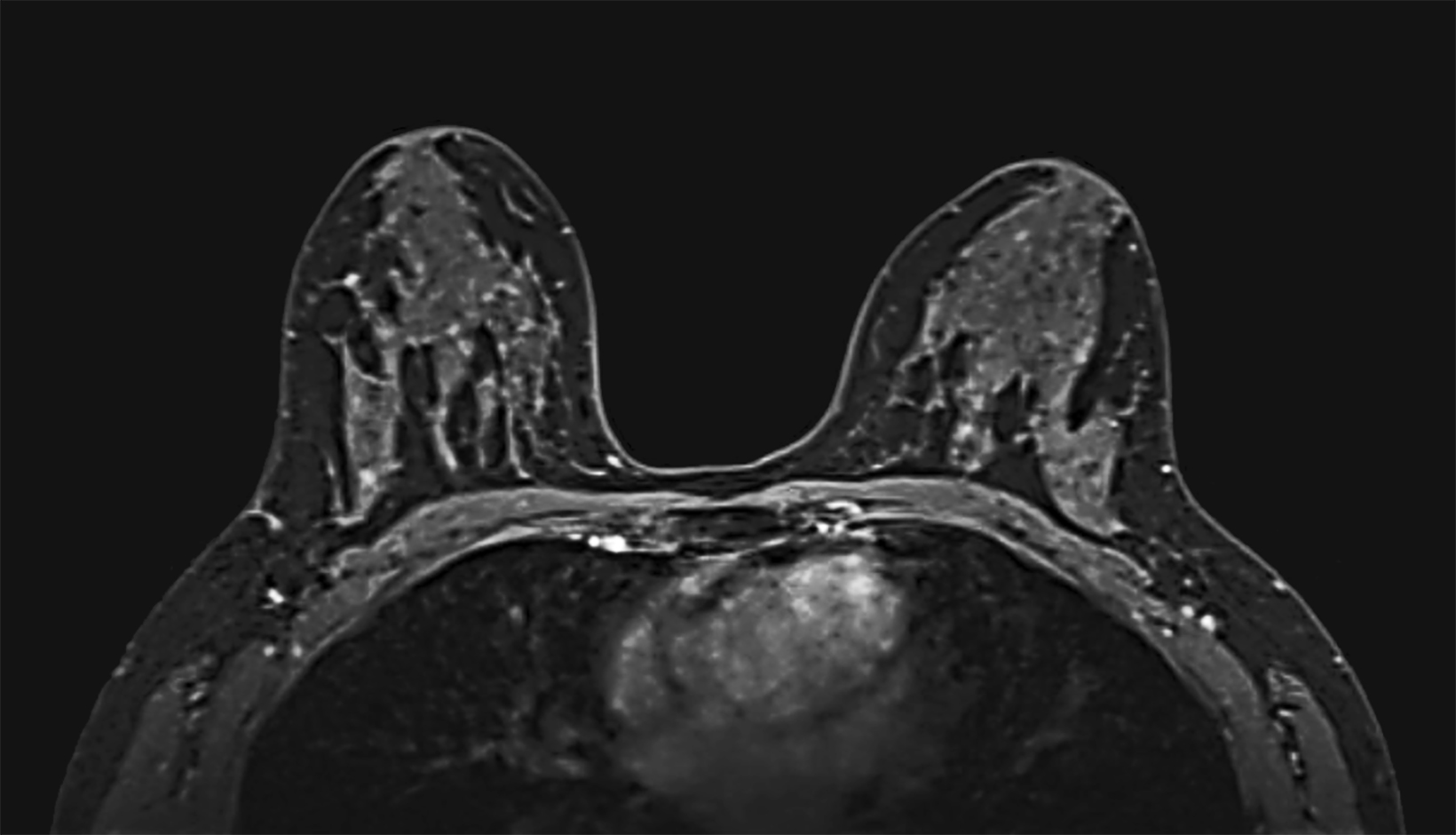
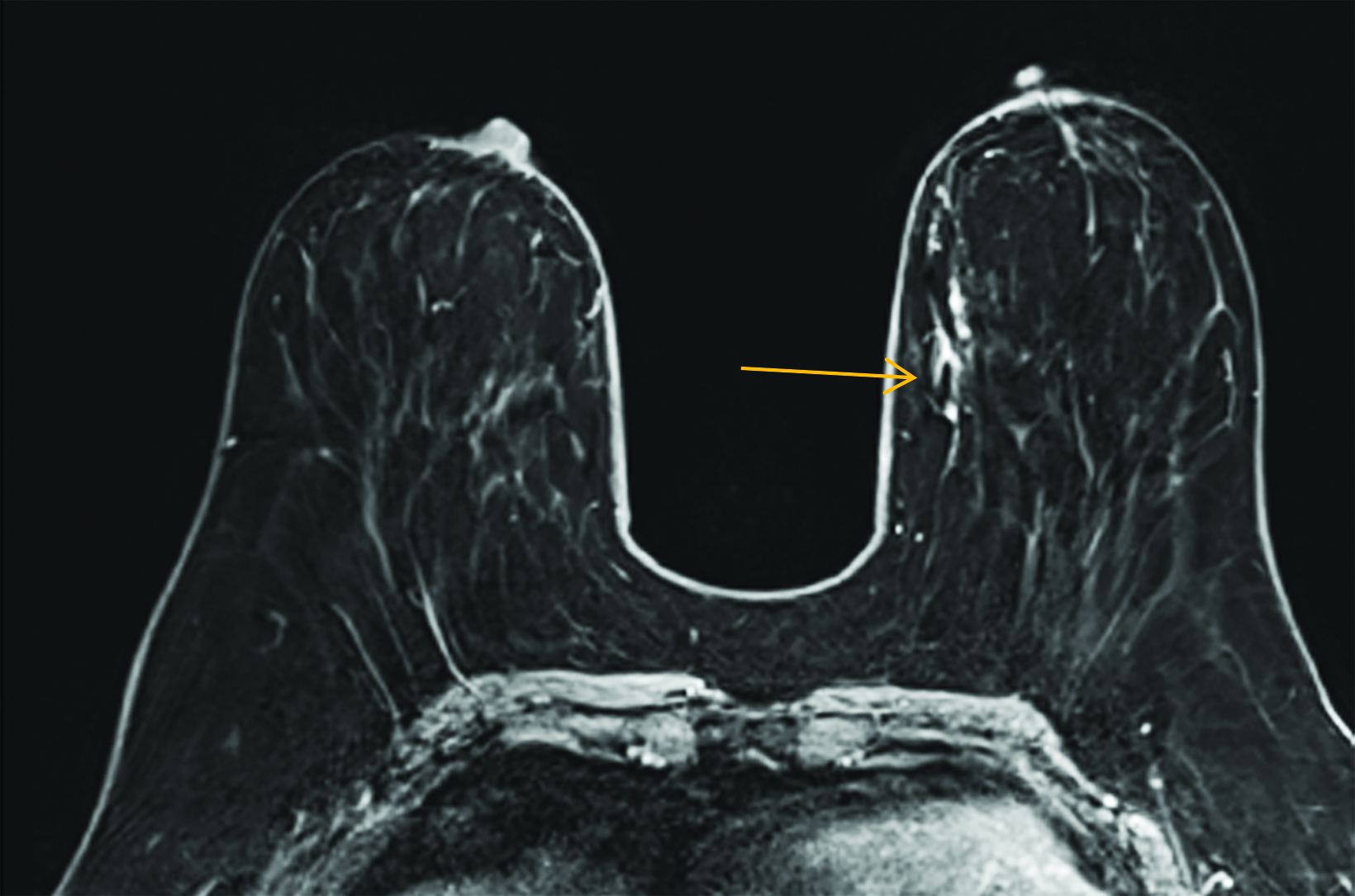
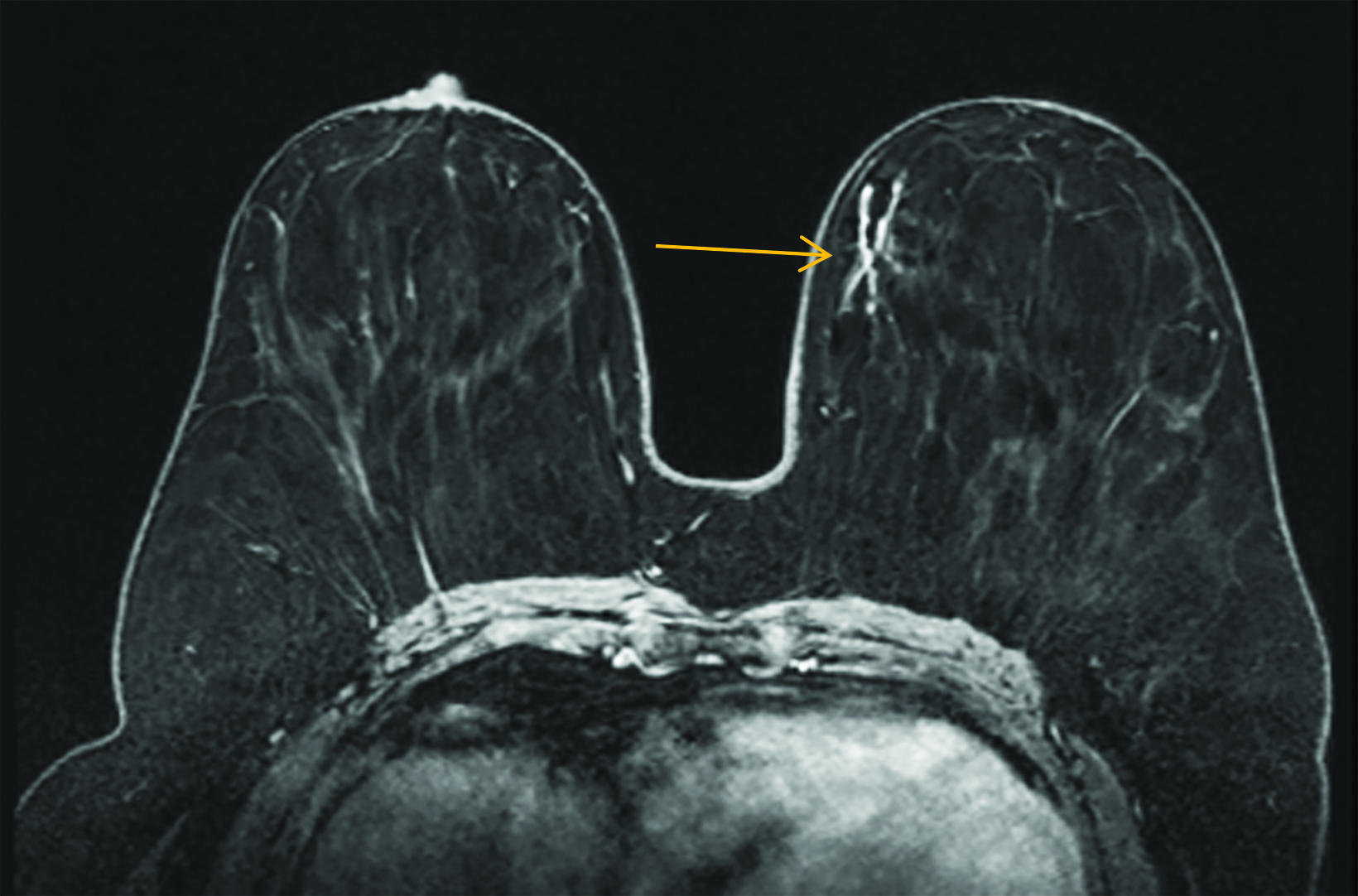
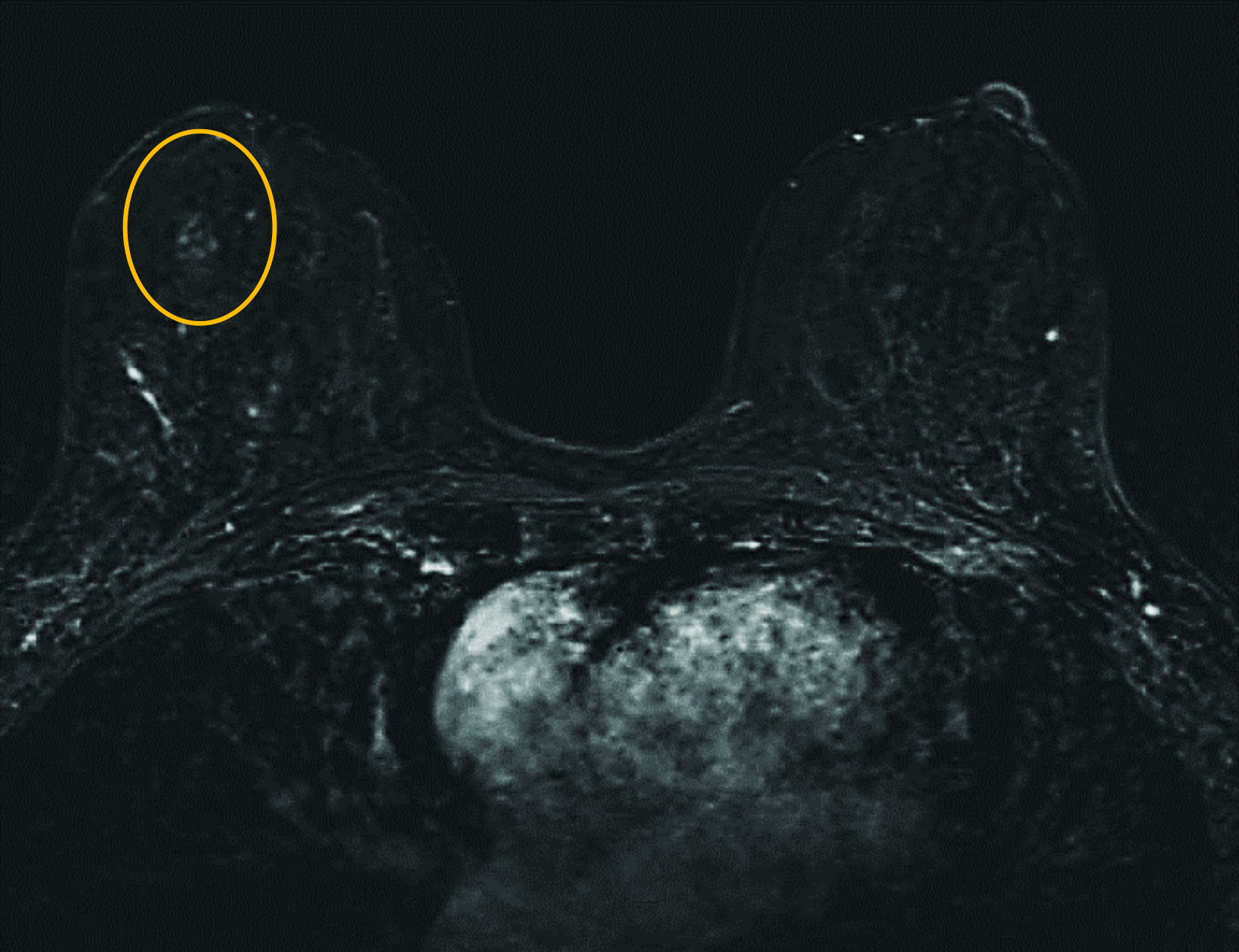
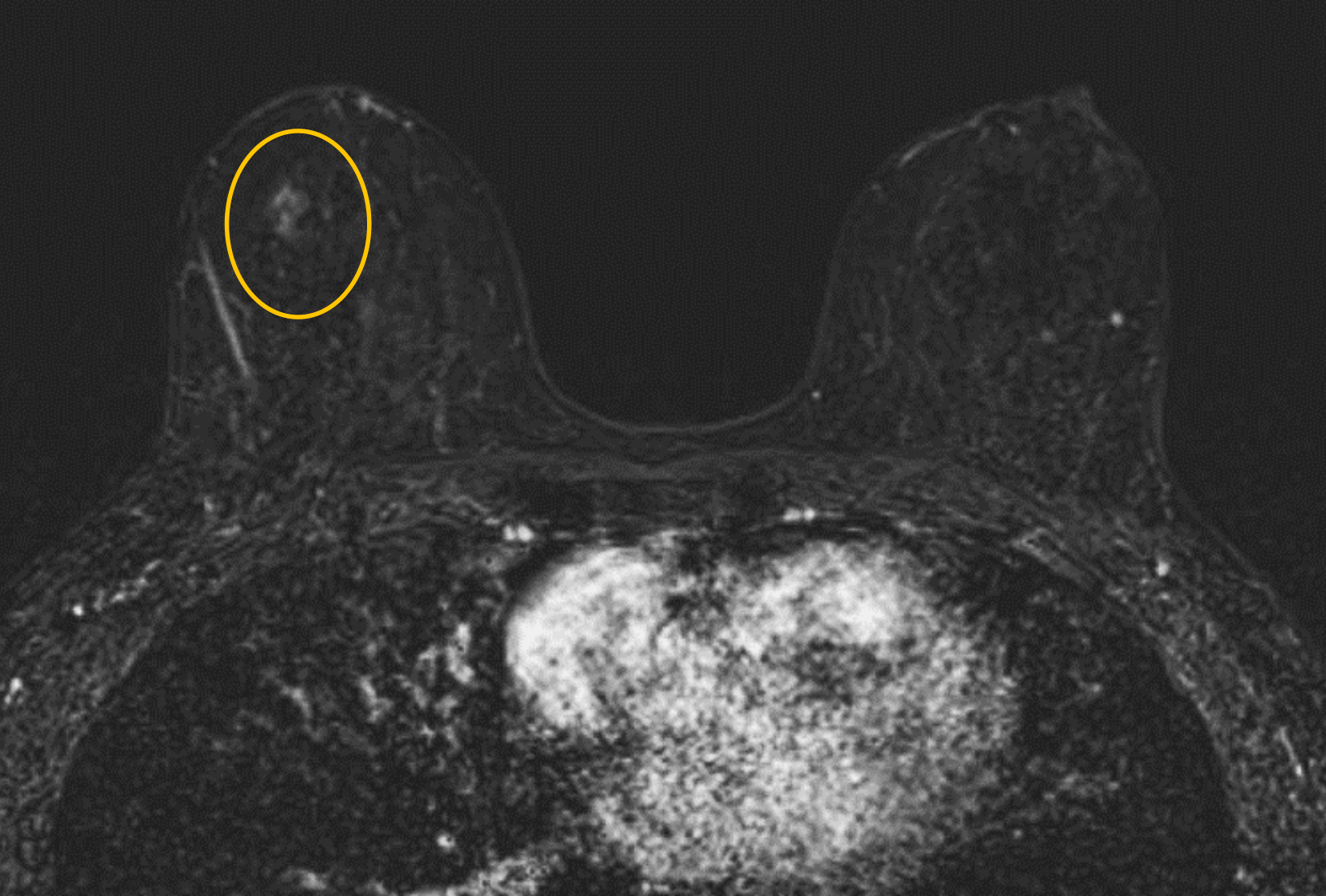
Our practice at the University of Texas Southwestern Medical Center has successfully introduced gadopiclenol in screening breast exams with minimal workflow interruption and protocol changes. Administering half the gadolinium dose with 0.05 mmol/kg gadopiclenol yields images with at least the same conspicuity and level of enhancement as that achieved with a full 0.1 mmol/kg dose of gadobutrol (Figures 1-3). By comparing breast exams from the same patient using different GBCAs, we have been able to gain a better understanding of how gadopiclenol behaves and how it can be leveraged to serve our patient population best.
Summary
Contrast-enhanced breast MRI is a mainstay in the diagnostic armamentarium for breast cancer detection and characterization. With its high sensitivity, multiplanar and multiparametric imaging capability, breast MRI can help guide clinical decision-making across various clinical scenarios. Despite safety considerations associated with GBCA administration, the benefits of contrast-enhanced breast MRI continue to outweigh the risks, offering invaluable insights into breast pathology and facilitating personalized patient care. Gadopiclenol is the latest macrocyclic GBCA with a robust safety profile and can help decrease the gadolinium dose needed for breast MRI.
VUEWAY® (gadopiclenol) solution for injection
Indications
VUEWAY injection is indicated in adults and children aged 2 years and older for use with magnetic resonance imaging (MRI) to detect and visualize lesions with abnormal vascularity in:
- the central nervous system (brain, spine and associated tissues),
- the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system).
IMPORTANT SAFETY INFORMATION
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
Risk Associated with Intrathecal Use
Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. VUEWAY is not approved for intrathecal use.
NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.
- The risk for NSF appears highest among patients with:
- Chronic, severe kidney disease (GFR < 30 mL/min/1.73 m2), or
- Acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension, diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- For patients at highest risk for NSF, do not exceed the recommended VUEWAY dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration.
Contraindications
VUEWAY injection is contraindicated in patients with history of hypersensitivity reactions to VUEWAY.
Warnings and Precautions
There are risks associated with intrathecal use of GBCAs that can cause serious adverse reactions including death, coma, encephalopathy, and seizures. The safety and effectiveness of VUEWAY have not been established with intrathecal use and VUEWAY is not approved for intrathecal use.
Risk of nephrogenic systemic fibrosis is increased in patients using GBCA agents that have impaired elimination of the drugs, with the highest risk in patients with chronic, severe kidney disease as well as patients with acute kidney injury. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities.
Hypersensitivity reactions including serious hypersensitivity reactions, could occur during use or shortly following VUEWAY administration. Assess all patients for any history of a reaction to contrast media, bronchial asthma and/or allergic disorders, administer VUEWAY only in situations where trained personnel and therapies are promptly available for the treatment of hypersensitivity reactions, and observe patients for signs and symptoms of hypersensitivity reactions afteradministration.
Gadolinium retention can be for months or years in several organs after administration. The highest concentrations (nanomoles per gram of tissue) have been identified in the bone, followed by other organs (brain, skin, kidney, liver and spleen). Minimize repetitive GBCA imaging studies, particularly closely spaced studies, when possible.
Acute kidney injury requiring dialysis has occurred with the use of GBCAs in patients with chronically reduced renal function. The risk of acute kidney injury may increase with increasing dose of the contrast agent.
Extravasation and injection site reactions can occur with administration of VUEWAY. Ensure catheter and venous patency before the injection of VUEWAY.
VUEWAY may impair the visualization of lesions seen on non-contrast MRI. Therefore, caution should be exercised when VUEWAY MRI scans are interpreted without a companion non-contrast MRI scan.
The most common adverse reactions (incidence ≥ 0.5%) are injection site pain (0.7%), and headache (0.7%).
POST-MARKETING EVENTS
Acute pancreatitis within 48 hours of GBCA administration has been reported.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for VUEWAY® (gadopiclenol) solution for injection including BOXED WARNING on Nephrogenic Systemic Fibrosis.
Manufactured for Bracco Diagnostics Inc. by Liebel-Flarsheim Company LLC, Raleigh, NC, USA 27616.
VUEWAY is a registered trademark of Bracco Imaging S.p.A.
Bracco Diagnostics Inc.
Prospect Road, Building H
Monroe Township, NJ 08831 USA
Phone: 609-514-2200
Toll-Free: 1-877-272-2269 (U.S. only)
Fax: 609-514-2446
©2024 Bracco Diagnostics Inc. All Rights Reserved.
References
- World Health Organization. International Agency for Research on Cancer. Global Cancer Observatory, Cancer Today GLOBOCAN 2022. Breast cancer fact sheet. Accessed April 17, 2024. https://gco.iarc.who.int/media/globocan/factsheets/cancers/20-breast-fact-sheet.pdf
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299(2):278-286.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404.
- Bendszus M, Laghi A, Munuera J, Tanenbaum LN, Taouli B, Thoeny HC. MRI gadolinium-based contrast media: meeting radiological, clinical, and environmental needs. J Magn Reson Imaging. Published online January 16, 2024.
- McDonald RJ, Levine D, Weinreb J, et al. Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology. 2018;289(2):517-534.
- VUEWAY® (gadopiclenol) solution for injection, 485.1 mg/mL. Full Prescribing Information and Medication Guide. Monroe Twp, NJ: Bracco Diagnostics Inc.; January 2024.
- Elucirem™ (gadopiclenol) injection. Full Prescribing Information. Princeton, NJ: Guerbet LLC.; September 2022.
- Sardanelli F, Cozzi A, Trimboli RM, Schiaffino S. Gadolinium retention and breast MRI screening: more harm than good? AJR Am J Roentgenol. 2020;214(2):324-327.
- American College of Radiology. ACR practice parameter for the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast. Revised 2023. Accessed March 31, 2024. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/MR-Contrast-Breast.pdf
- Kanal E, Maravilla K, Rowley HA. Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol. 2014;35(12):2215-2226.
- Martincich L, Faivre-Pierret M, Zechmann CM, et al. Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for breast MR imaging (DETECT Trial). Radiology. 2011;258(2):396-408.
- Robic C, Port M, Rousseaux O, et al. Physicochemical and pharmacokinetic profiles of gadopiclenol: a new macrocyclic gadolinium chelate with high T1 relaxivity. Invest Radiol. 2019;54(8):475-484.
- American College of Radiology (ACR) Committee on Drugs and Contrast Media. ACR Manual on Contrast Media, Version 2023. Accessed April 1, 2024. https://www. acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
- Idée JM, Port M, Robic C, Medina C, Sabatou M, Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging. 2009;30:1249-1258.
- Kanal E. Gadolinium based contrast agents (GBCA): safety overview after 3 decades of clinical experience. Magn Reson Imaging. 2016;34(10):1341-1345.
- ProHance® (Gadoteridol) Injection, 279.3 mg/mL. Full Prescribing Information and Medication Guide. Monroe Twp, NJ: Bracco Diagnostics Inc.; January 2024.
- Gadavist® (gadobutrol) injection. Full Prescribing Information. Whippany, NJ: Bayer Healthcare Pharmaceuticals; April 2022.
- Dotarem® (gadoterate meglumine) injection. Full Prescribing Information. Princeton, NJ: Guerbet LLC.; April 2022.
- Clariscan™ (gadoterate meglumine) injection. Full Prescribing Information. Marlborough, MA: GE Healthcare Inc.; November 2020.
- MultiHance® (gadobenate dimeglumine) injection, 529 mg/mL. Full Prescribing Information and Medication Guide. Bracco Diagnostics Inc. Monroe Township, NJ; January 2024.
- Kuhl C, Csőszi T, Piskorski W, Miszalski T, Lee JM, Otto PM. Efficacy and safety of half-dose gadopiclenol versus full-dose gadobutrol for contrast-enhanced body MRI. Radiology. 2023;308(1):e222612.
