Clinical Applications of Gadopiclenol in Body MRI
SPONSORED BY BRACCO
Introduction
Magnetic resonance imaging (MRI) is an essential imaging technique that enables disease diagnosis and guides treatment. Gadolinium- based contrast agents (GBCAs) are the most commonly used media to enhance the diagnostic ability of MRI. As with any medical intervention, the administration of a GBCA must undergo a careful risk-benefit analysis that considers a patient’s clinical presentation, the need for contrast, as well as the properties of the selected GBCA. While the current commercially available GBCAs carry an excellent safety profile,1 two areas of consideration are nephrogenic systemic fibrosis (NSF) and gadolinium retention. Such considerations are reflected in the development of agents with lower gadolinium dosing, the most recently approved of which is gadopiclenol.2 This article discusses considerations relevant to GBCA use for body MRI applications, with a focus on gadopiclenol.
Background: GBCA Use in MRI
GBCAs contain gadolinium in the form of a paramagnetic metal ion (Gd3+), which alters tissue magnetic properties to enable the visualization of certain pathological conditions that may be otherwise challenging or impossible to detect.3,4 In simple terms, the presence of GBCA within the tissue environment results in T1 shortening, increasing the tissue’s signal intensity on T1-weighted MRI.5 How bright a tissue appears on a given MRI pulse sequence after administration of GBCA depends on several factors, including but not limited to the tissue’s inherent properties, MRI magnet field strength, image acquisition parameters, timing of image acquisition after GBCA administration, and physiochemical properties of the GBCA. A fundamental property of the GBCA that determines tissue brightness is its intrinsic relaxivity, which reflects a change in the solution relaxation rate as a function of GBCA concentration. GBCAs often affect both r1 and r2 relaxivity. Agents with higher r1 relaxivity have been demonstrated to have a favorable performance for detection of enhancing lesions.4 Hence, the ideal GBCA would have both high relaxivity (to optimize diagnostic performance) and tolerability (to maximize safety).
While the current commercially available GBCAs exhibit differences in physiochemical properties and tissue distribution (Table 1), they are generally well tolerated; adverse reactions are rare, and most are mild and self-limiting.3,6-13 Moreover, proper screening of at-risk patients and the availability of more stable GBCAs have significantly decreased the risk of serious adverse reactions such as NSF. Although the associated risk of NSF with GBCAs is established, NSF is exceedingly rare and only a few cases have been reported after 2008.14 Another topic of recent interest is gadolinium retention in healthy tissue among those with normal kidney function.
Gadolinium retention occurs to some extent with all GBCAs, but as macrocyclic agents exhibit faster clearance, it is more pronounced with linear agents and after repeated administrations.15 However, no currently available data support a link between gadolinium retention and adverse clinical effects, even among linear GBCAs with the relatively highest rates of retention.3,16-20 Naturally, the ideal GBCA would simultaneously provide high relaxivity (allowing for administration of small doses), high structural stability (minimizing potential for gadolinium release), and minimal adverse clinical effects.
Gadopiclenol for Contrast-Enhanced Body MRI
Gadopiclenol is the most recent addition to the class of US Food and Drug Administration (FDA)-approved GBCAs. This macrocyclic molecule offers approximately two to three times higher relaxivity than the other commercially available extracellular fluid GBCAs. The higher relaxivity enables administration of a lower gadolinium dosage of 0.05 mmol/kg (compared with 0.1 mmol/kg for other extracellular fluid agents approved with similar indications in the US).12,13,21
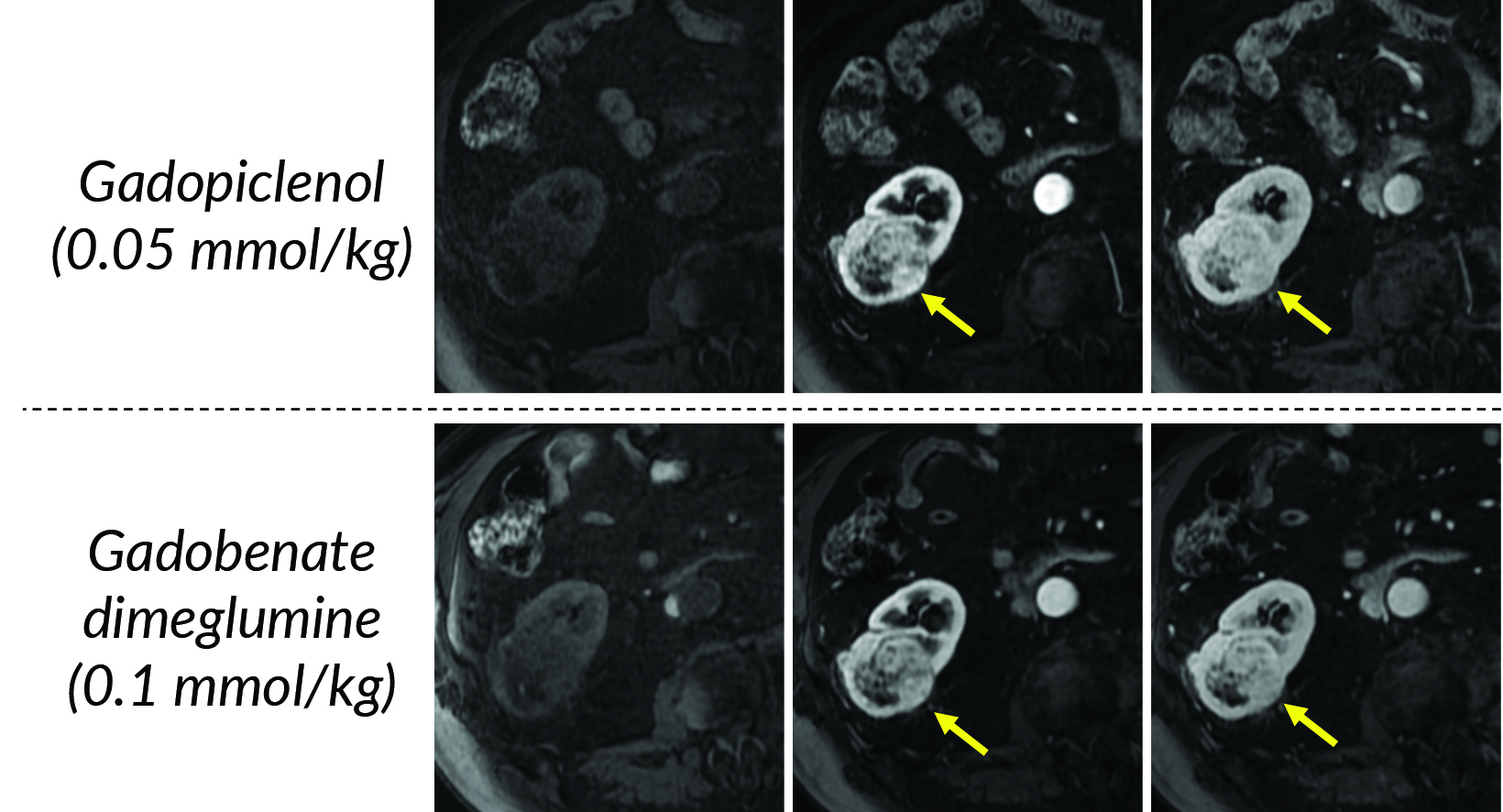
In terms of clinical utility in body MRI, the efficacy and safety of gadopiclenol was evaluated in a phase 3 study where gadopiclenol at the dose of 0.05 mmol/kg was compared with gadobutrol at the dose of 0.1 mmol/kg in 273 patients who underwent MRI of different body regions.22 The body regions comprised the abdomen (including the liver, pancreas, and kidneys) in 35% of the patients, pelvis (including the uterus, ovary, and prostate) in 22% of patients, head and neck in 8% of patients, breast or thorax for 28% of patients, and the musculoskeletal system in 8% of patients. Considering that gadopiclenol was administered at only half the gadolinium dose of gadobutrol, there was no preference between gadopiclenol-enhanced images and gadobutrol-enhanced images in 75% to 83% of the cases. When a preference was reported, 12% to 15% were in favor of gadopiclenol while 5.5% to 11% were in favor of gadobutrol. The quantitative analysis indicated that the enhancement percentage was significantly higher with gadopiclenol than with gadobutrol for two of the three reading groups, but no difference was noted in the lesion-to-background enhancement ratio between the two agents. Moreover, there was no difference in the rate, intensity, or type of adverse events between the two agents. Hence, it was concluded that gadopiclenol at 0.05 mmol/kg was comparable to gadobutrol at 0.1 mmol/kg with respect to lesion visualization and safety.22
Selecting a GBCA for Body MRI Applications
The selection of a GBCA for body MRI applications involves careful consideration of several factors, including the GBCA’s diagnostic performance and its functionality specific to the clinical indication being evaluated.6-13 Each GBCA possesses unique properties that can influence efficacy in various clinical scenarios (Table 1). For example, while extracellular fluid GBCAs with high relaxivity can provide excellent depiction of hypervascular lesions as well as anatomic delineation of the majority of the organs in the abdomen and pelvis, hepatocyte-specific contrasts are often preferred for characterizing lesions with possible functioning hepatocytes, evaluating the biliary system, or detecting metastases in the liver through high-resolution delayed postcontrast imaging.23 Nearly all contrast-enhanced body MRI examinations are performed with either an extracellular or a hepatocyte-specific GBCA. Several factors weigh into the choice of the extracellular GBCA (several of which depend on regional/institutional practices), such as relaxivity, structure (linear vs macrocyclic), cost, radiologist experience/preference, etc. Moreover, there is a growing emphasis on adopting strategies to help minimize gadolinium exposure while preserving diagnostic efficacy—a concept similar to ALARA (as low as reasonably achievable) in utilizing ionizing radiation to achieve diagnostic images.24 As noted by the American College of Radiology for GBCAs, group II agents should only be administered if deemed necessary, and in general, the lowest dose needed for diagnosis should be used, including for at-risk patients in whom the dose should not exceed the recommended single dose.3< Hence, when the relaxivity and resulting efficacy of two GBCAs are comparable, opting for a more stable agent with lower gadolinium exposure appears to be the most reasonable decision.
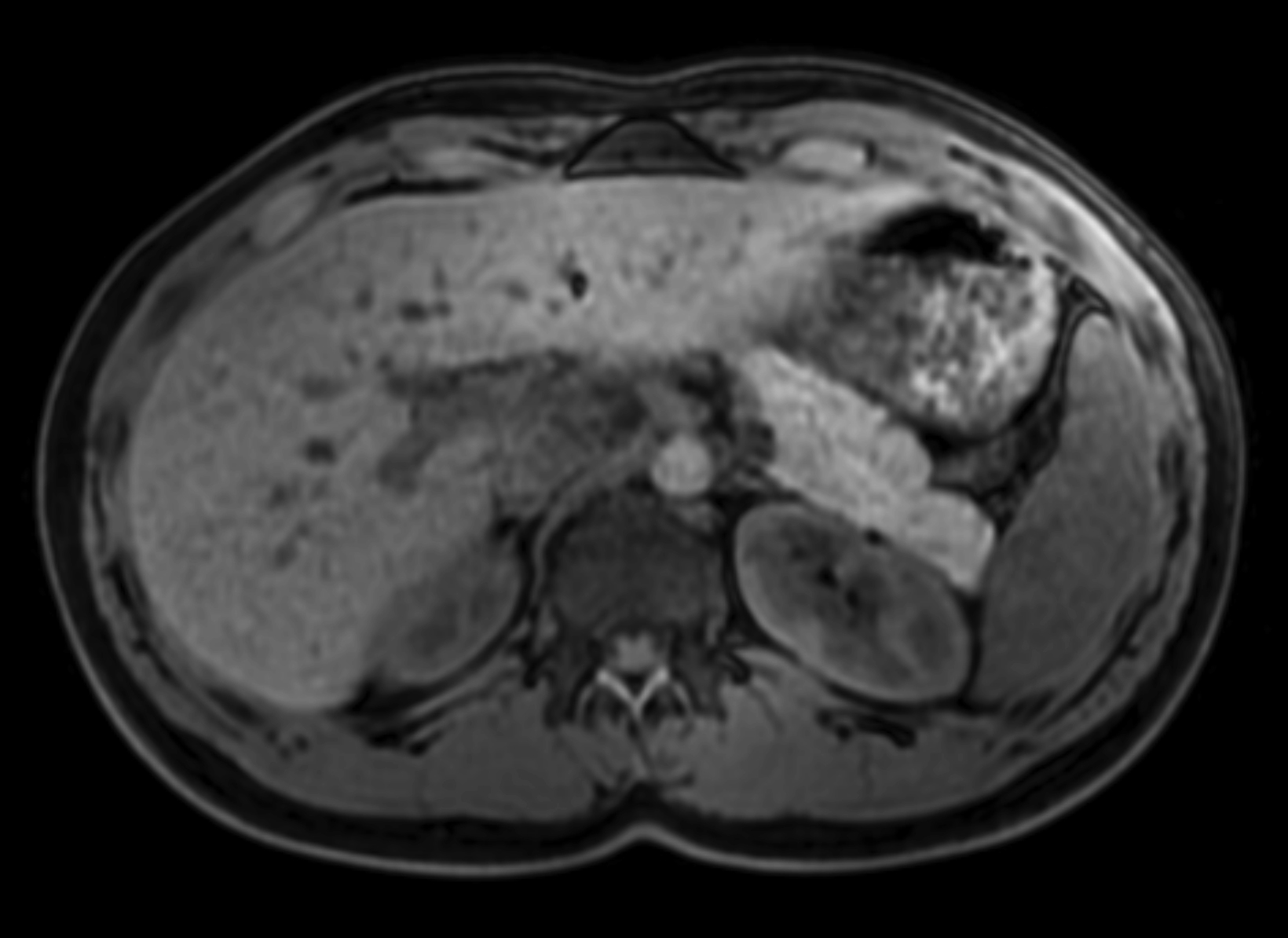
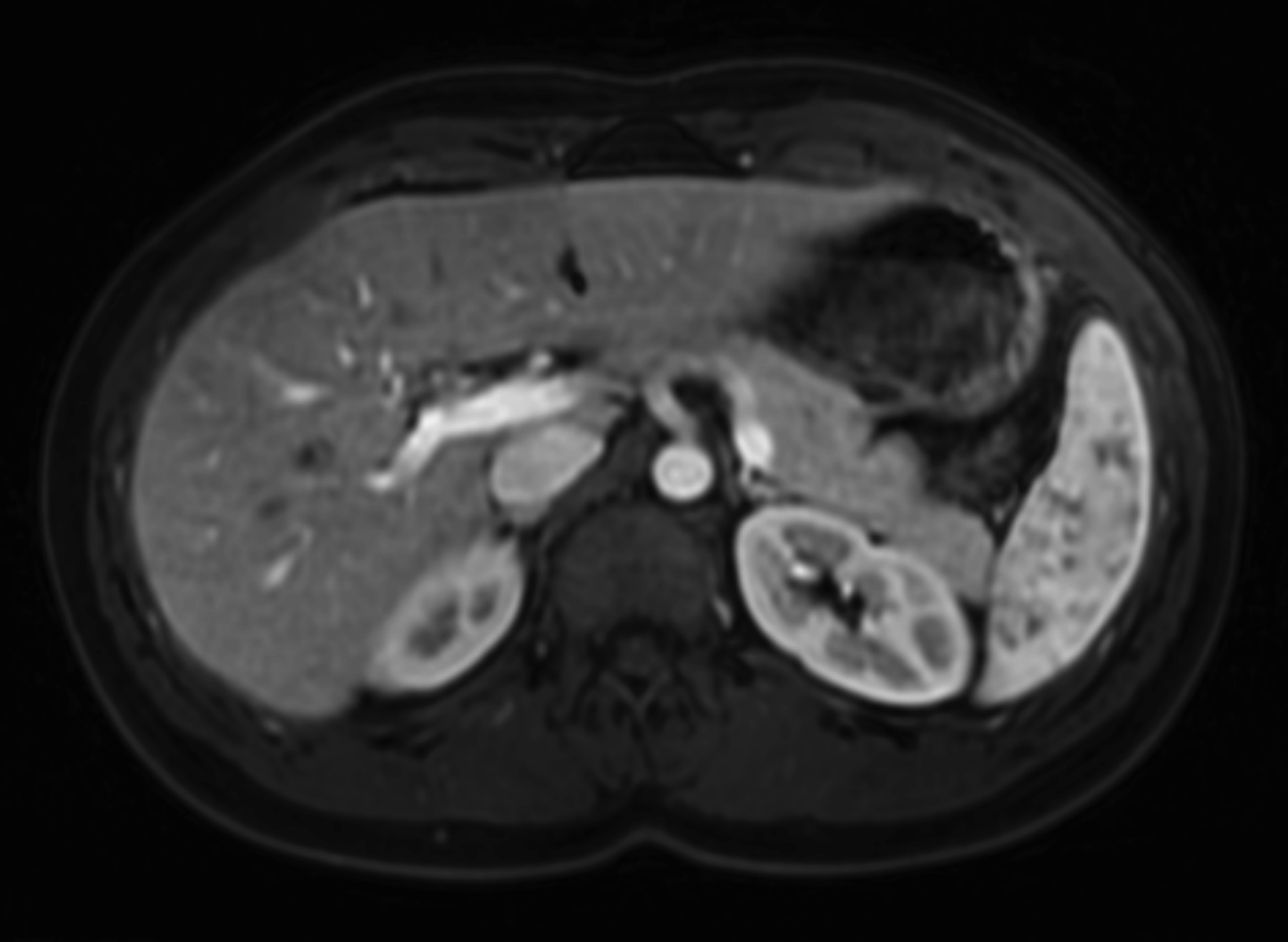
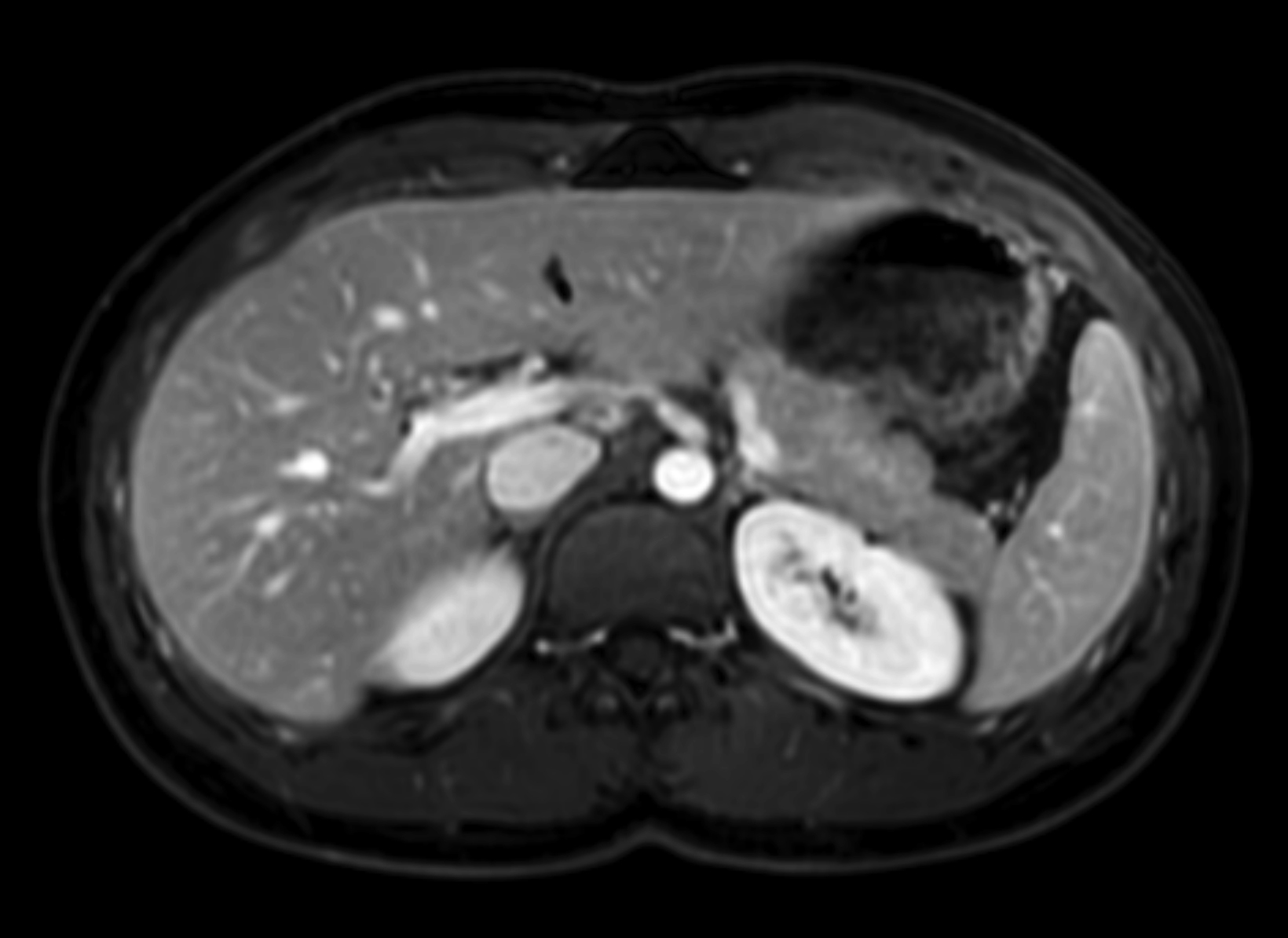
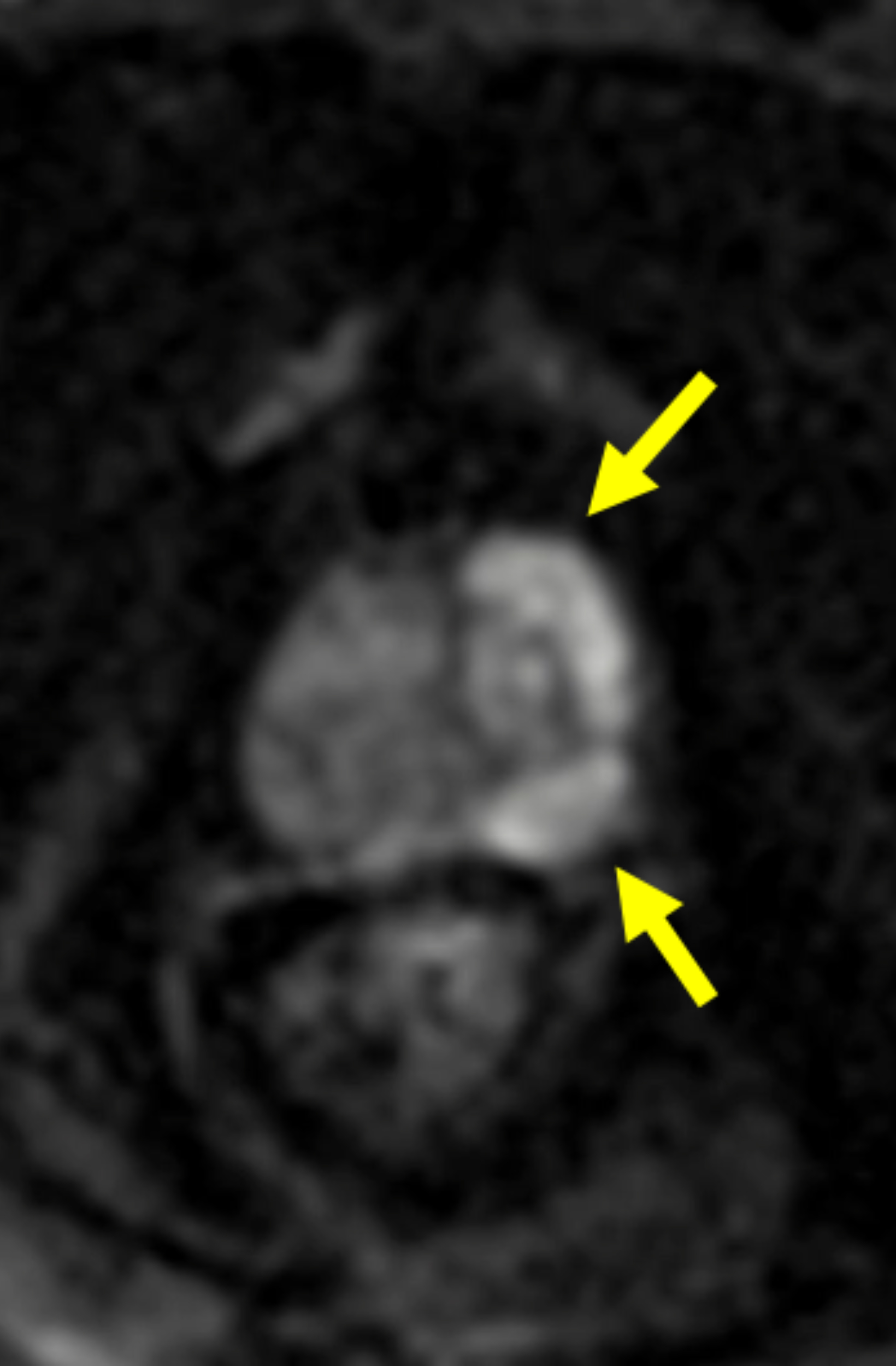
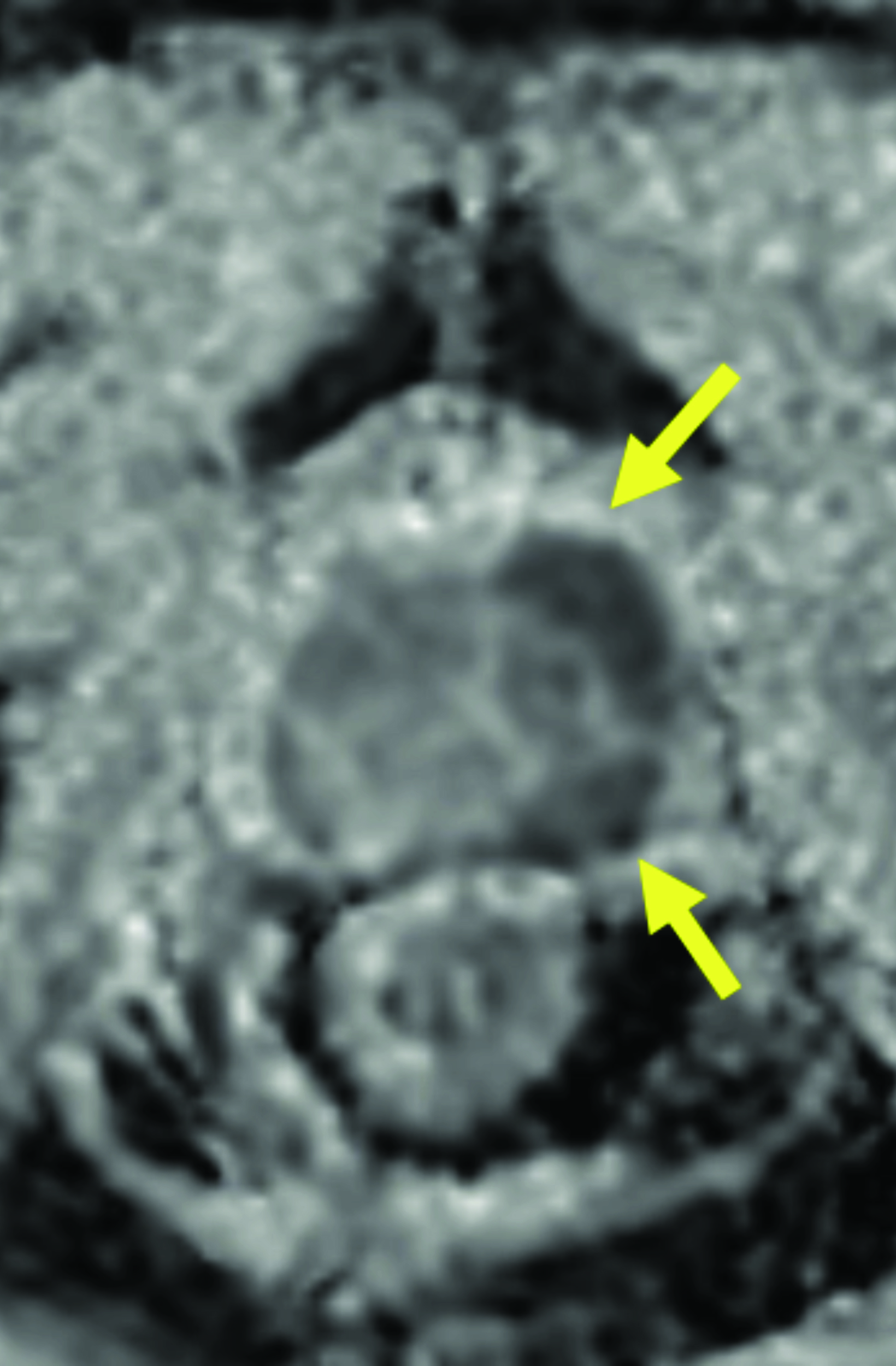
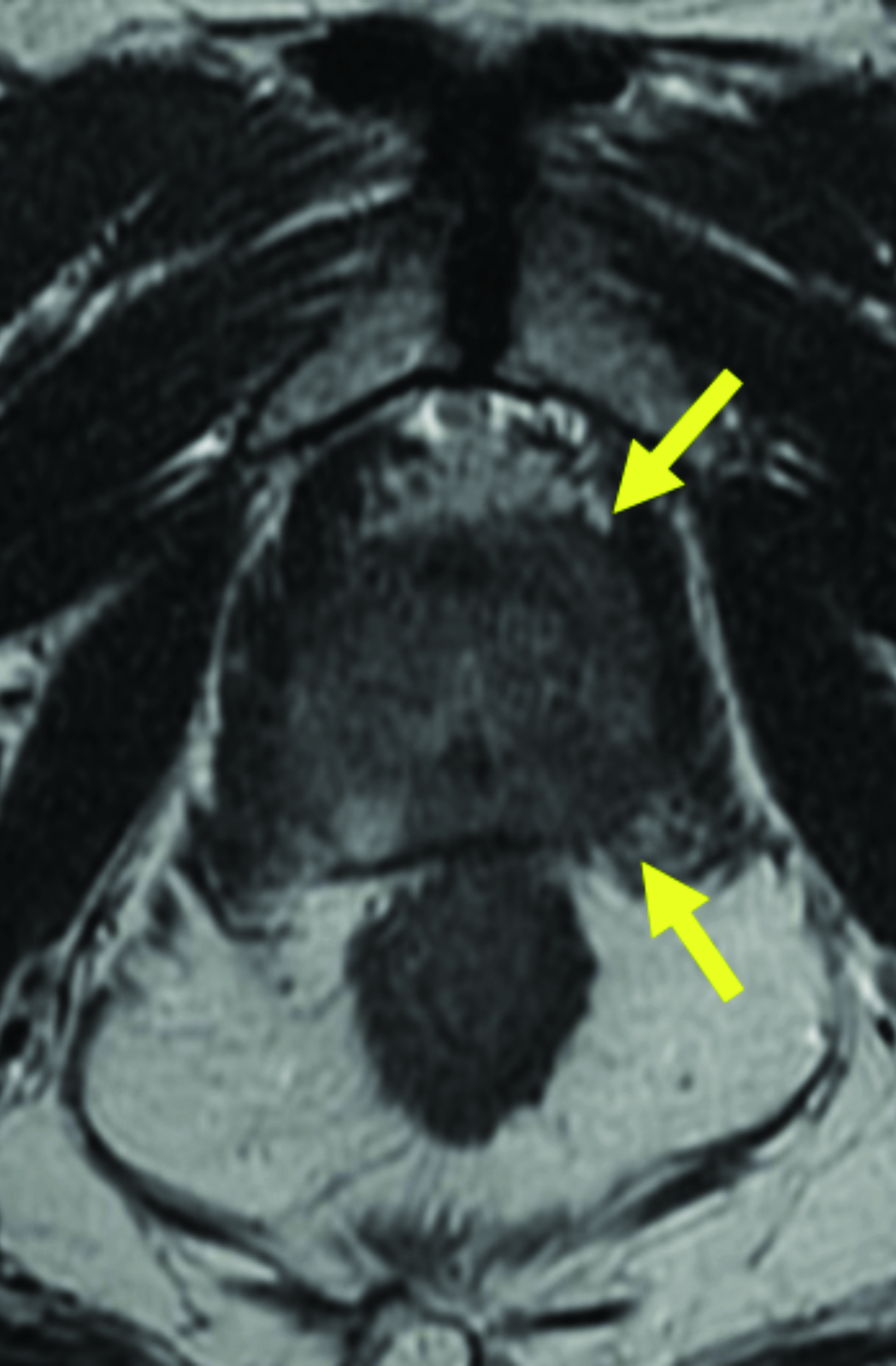
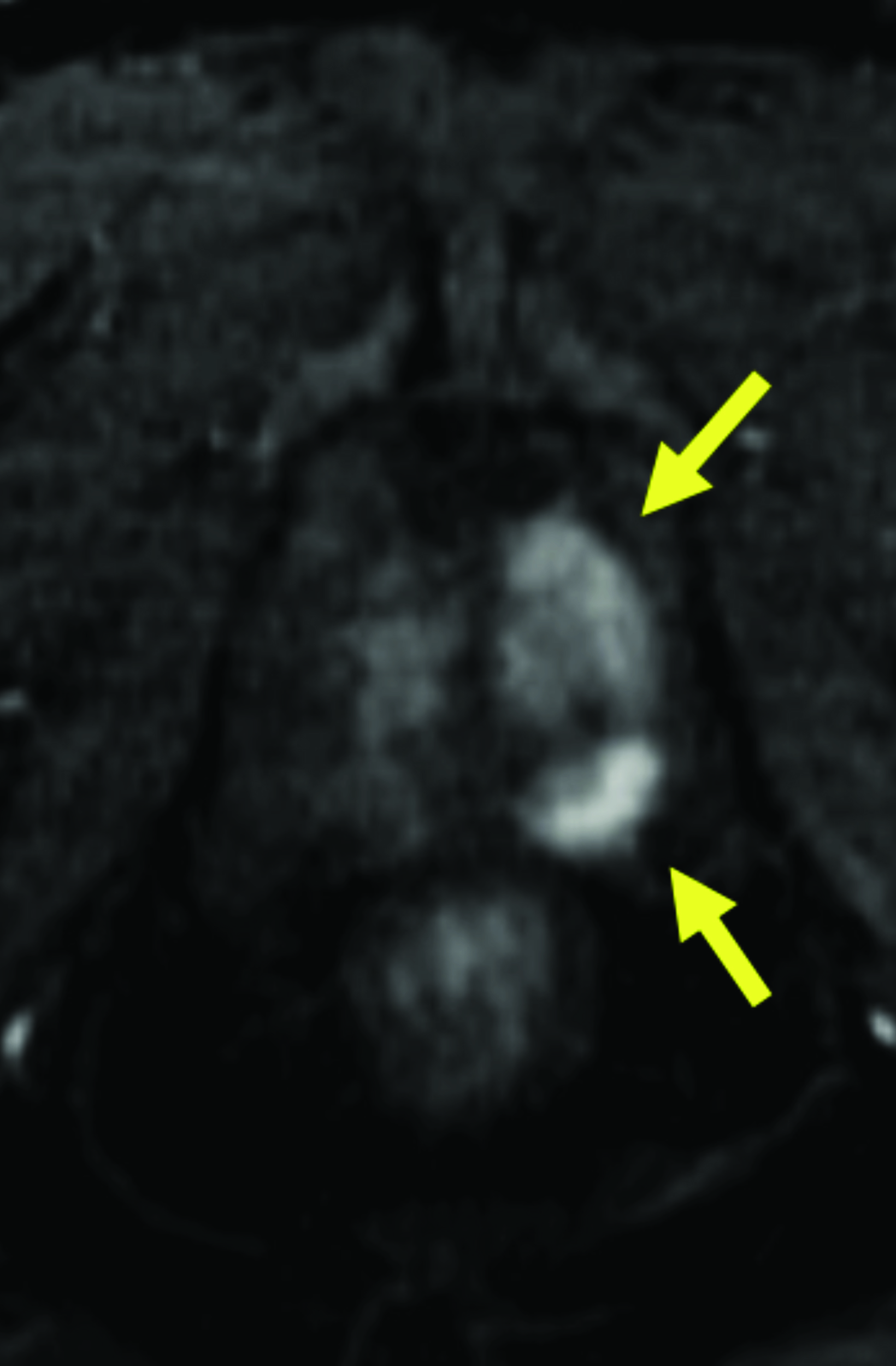
Given its high relaxivity, macrocyclic structure, and half the amount of gadolinium exposure compared with the other extracellular GBCAs, gadopiclenol may appear as the natural choice for the extracellular agent used in body MRI applications. However, even if this is the case, adopting a new GBCA in a clinical practice requires crossing several hurdles, including introduction to the institution’s formulary, technologist/radiologist education, internal diagnostic performance validation, cost analysis, and rate of adverse events. Our practice has successfully implemented gadopiclenol as the extracellular GBCA for all body MRI applications, achieving essentially the same image quality as before without the need for major changes in protocols or workflow. Gadopiclenol has not negatively impacted dynamic contrast-enhanced imaging at our institution (Figures 1-3), which is significant in terms of generating high-quality diagnostic images and maintaining a consistent workflow with little-to-no change for the imaging technologists.
Summary
GBCA-enhanced body MRI continues to play a pivotal role in disease diagnosis and management. Gadopiclenol exposes patients to half the gadolinium, due to its approximately two to three times higher relaxivity versus other current commercially available extracellular agents. This macrocyclic agent also provides image quality comparable to that of other agents. These benefits position gadopiclenol as an attractive extracellular agent for body MRI applications. Our institutional experience with implementing gadopiclenol for body MRI has been rather straightforward; through appropriate technologist/radiologist education and using a team-based approach to internal image quality validation, we have maintained high-quality body MRI image acquisition with no major change in workflow.
VUEWAY® (gadopiclenol) solution for injection
Indications
VUEWAY injection is indicated in adults and children aged 2 years and older for use with magnetic resonance imaging (MRI) to detect and visualize lesions with abnormal vascularity in:
- the central nervous system (brain, spine and surrounding tissues),
- the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system).
IMPORTANT SAFETY INFORMATION
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
Risk Associated with Intrathecal Use
Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. VUEWAY is not approved for intrathecal use.
NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.
- The risk for NSF appears highest among patients with:
- Chronic, severe kidney disease (GFR < 30 mL/min/1.73 m2), or
- Acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension, diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- For patients at highest risk for NSF, do not exceed the recommended VUEWAY dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration.
Contraindications
VUEWAY injection is contraindicated in patients with history of hypersensitivity reactions to VUEWAY.
Warnings and Precautions
There are risks associated with intrathecal use of GBCAs that can cause serious adverse reactions including death, coma, encephalopathy, and seizures. The safety and effectiveness of VUEWAY have not been established with intrathecal use and VUEWAY is not approved for intrathecal use.
Risk of nephrogenic systemic fibrosis is increased in patients using GBCA agents that have impaired elimination of the drugs, with the highest risk in patients with chronic, severe kidney disease as well as patients with acute kidney injury. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities.
Hypersensitivity reactions including serious hypersensitivity reactions, could occur during use or shortly following VUEWAY administration. Assess all patients for any history of a reaction to contrast media, bronchial asthma and/or allergic disorders, administer VUEWAY only in situations where trained personnel and therapies are promptly available for the treatment of hypersensitivity reactions, and observe patients for signs and symptoms of hypersensitivity reactions after administration.
Gadolinium retention can be for months or years in several organs after administration. The highest concentrations (nanomoles per gram of tissue) have been identified in the bone, followed by other organs (brain, skin, kidney, liver and spleen). Minimize repetitive GBCA imaging studies, particularly closely spaced studies, when possible.
Acute kidney injury requiring dialysis has occurred with the use of GBCAs in patients with chronically reduced renal function. The risk of acute kidney injury may increase with increasing dose of the contrast agent.
Extravasation and injection site reactions can occur with administration of VUEWAY. Ensure catheter and venous patency before the injection of VUEWAY.
VUEWAY may impair the visualization of lesions seen on non-contrast MRI. Therefore, caution should be exercised when VUEWAY MRI scans are interpreted without a companion non-contrast MRI scan.
The most common adverse reactions (incidence ≥ 0.5%) are injection site pain (0.7%), and headache (0.7%).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please obtain full Prescribing Information for VUEWAY® (gadopiclenol) solution for injection including BOXED WARNING on Nephrogenic Systemic Fibrosis at https://www.bracco.com/sites/default/files/2022-11/us-en-spc-vueway.pdf
Manufactured for Bracco Diagnostics Inc. by Liebel-Flarsheim Company LLC, Raleigh, NC, USA 27616.
VUEWAY is a registered trademark of Bracco Imaging S.p.A.
Bracco Diagnostics Inc.
Prospect Road, Building H
Monroe Township, NJ 08831 USA
Phone: 609-514-2200
Toll-Free: 1-877-272-2269 (U.S. only)
Fax: 609-514-2446
©2024 Bracco Diagnostics Inc. All Rights Reserved.
References
- References
- Starekova J, Pirasteh A, Reeder SB. Update on gadolinium based contrast agent safety, from the AJR special series on contrast media. Am J Roentgenol. Published online October 18, 2023. doi:10.2214/AJR.23.30036
- Alsogati E, Ghandourah H, Bakhsh A. Review of the efficacy and safety of gadopiclenol: a newly emerging gadolinium-based contrast agent. Cureus. 2023;15(8):e43055.
- American College of Radiology (ACR) Committee on Drugs and Contrast Media. ACR Manual on Contrast Media, Version 2023. Accessed February 1, 2024. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
- Kanal E, Maravilla K, Rowley HA. Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol. 2014;35(12):2215-2226.
- Wahsner J, Gale EM, Rodríguez-Rodríguez A, Caravan P. Chemistry of MRI contrast agents: current challenges and new frontiers. Chem Rev. 2019;119(2):957-1057.
- MultiHance® (gadobenate dimeglumine) injection, 529 mg/mL. Full Prescribing Information and Medication Guide. Bracco Diagnostics Inc. Monroe Township, NJ; January 2024.
- Eovist® (gadoxetate disodium). Full Prescribing information. Whippany, NJ: Bayer Healthcare Pharmaceuticals; December 2021.
- ProHance® (Gadoteridol) Injection, 279.3 mg/mL. Full Prescribing Information and Medication Guide. Monroe Twp, NJ: Bracco Diagnostics Inc.; January 2024.
- Gadavist® (gadobutrol) injection. Full Prescribing Information. Whippany, NJ: Bayer Healthcare Pharmaceuticals; April 2022.
- Dotarem® (gadoterate meglumine) injection. Full Prescribing Information. Princeton, NJ: Guerbet LLC.; April 2022.
- Clariscan™ (gadoterate meglumine) injection. Full Prescribing Information. Marlborough, MA: GE Healthcare Inc.; November 2020.
- VUEWAY® (gadopiclenol) solution for injection, 485.1 mg/mL. Full Prescribing Information and Medication Guide. Monroe Twp, NJ: Bracco Diagnostics Inc.; January 2024.
- Elucirem™ (gadopiclenol) injection. Full Prescribing Information. Princeton, NJ: Guerbet LLC.; September 2022.
- Attari H, Cao Y, Elmholdt TR, Zhao Y, Prince MR. A systematic review of 639 patients with biopsy-confirmed nephrogenic systemic fibrosis. Radiology. 2019;292(2):376-386.
- McDonald RJ, Levine D, Weinreb J, et al. Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA Workshop on gadolinium chelates. Radiology. 2018;289(2):517-534.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276(1):228-232.
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275(3):772-782.
- Murata N, Gonzalez-Cuyar LF, Murata K, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. 2016;51(7):447-453.
- Parillo M, Sapienza M, Arpaia F, et al. A structured survey on adverse events occurring within 24 hours after intravenous exposure to gadodiamide or gadoterate meglumine: a controlled prospective comparison study. Invest Radiol. 2019;54(4):191-197.
- Layne KA, Raja K, Dargan PI, Wood DM. Gadolinium concentrations in biological matrices from patients exposed to gadolinium-based contrast agents. Invest Radiol. 2021;56(7):458-464.
- Robic C, Port M, Rousseaux O, et al. Physicochemical and pharmacokinetic profiles of gadopiclenol: a new macrocyclic gadolinium chelate with high T1 relaxivity. Invest Radiol. 2019;54(8):475-484.
- Kuhl C, Csőszi T, Piskorski W, Miszalski T, Lee JM, Otto PM. Efficacy and safety of half-dose gadopiclenol versus full-dose gadobutrol for contrast-enhanced body MRI. Radiology. 2023;308(1):e222612.
- Donato H, França M, Candelária I, Caseiro-Alves F. Liver MRI: from basic protocol to advanced techniques. Eur J Radiol. 2017;93:30-39.
- Bendszus M, Laghi A, Munuera J, Tanenbaum LN, Taouli B, Thoeny HC. MRI gadolinium-based contrast media: meeting radiological, clinical, and environmental needs. J Magn Reson Imaging. Published online January 16, 2024.
- Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann H-J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715-724.
Affiliation: Department of Radiology, University of Wisconsin, Madison, WI
Disclosure: Dr Pirasteh discloses research support from GE Healthcare and Bracco Diagnostics
