Bronchoesophageal Fistula
By Zarour C, Ibrahim R, Mian A, Al-Hameed M, Ogunde, B, Gerasymchuk G
Case Summary
An adult with a 20 pack-year history of smoking and essential hypertension presented to the emergency department with complaints of intermittent, sharp, nonradiating epigastric and substernal pain of one month’s duration. Other symptoms included increasing dysphagia, decreased appetite, and 10 lb weight loss over one month. There was no alleviation with antacids. The patient denied fever, night sweats, and shortness of breath. Initial laboratory evaluation showed leukocytosis at approximately 14,000; hyponatremia at 133; and hypochloremia at 96. An electrocardiogram demonstrated sinus tachycardia with possible left atrial enlargement and left ventricular hypertrophy. Troponins were negative.
Imaging Findings
Initial chest radiography was unremarkable. Esophagogastroduodenoscopy and esophagogram confirmed the diagnosis of an ulcerated, annular mass in the esophagus with fistulous communication to the medial segment bronchus of the right lower lobe.
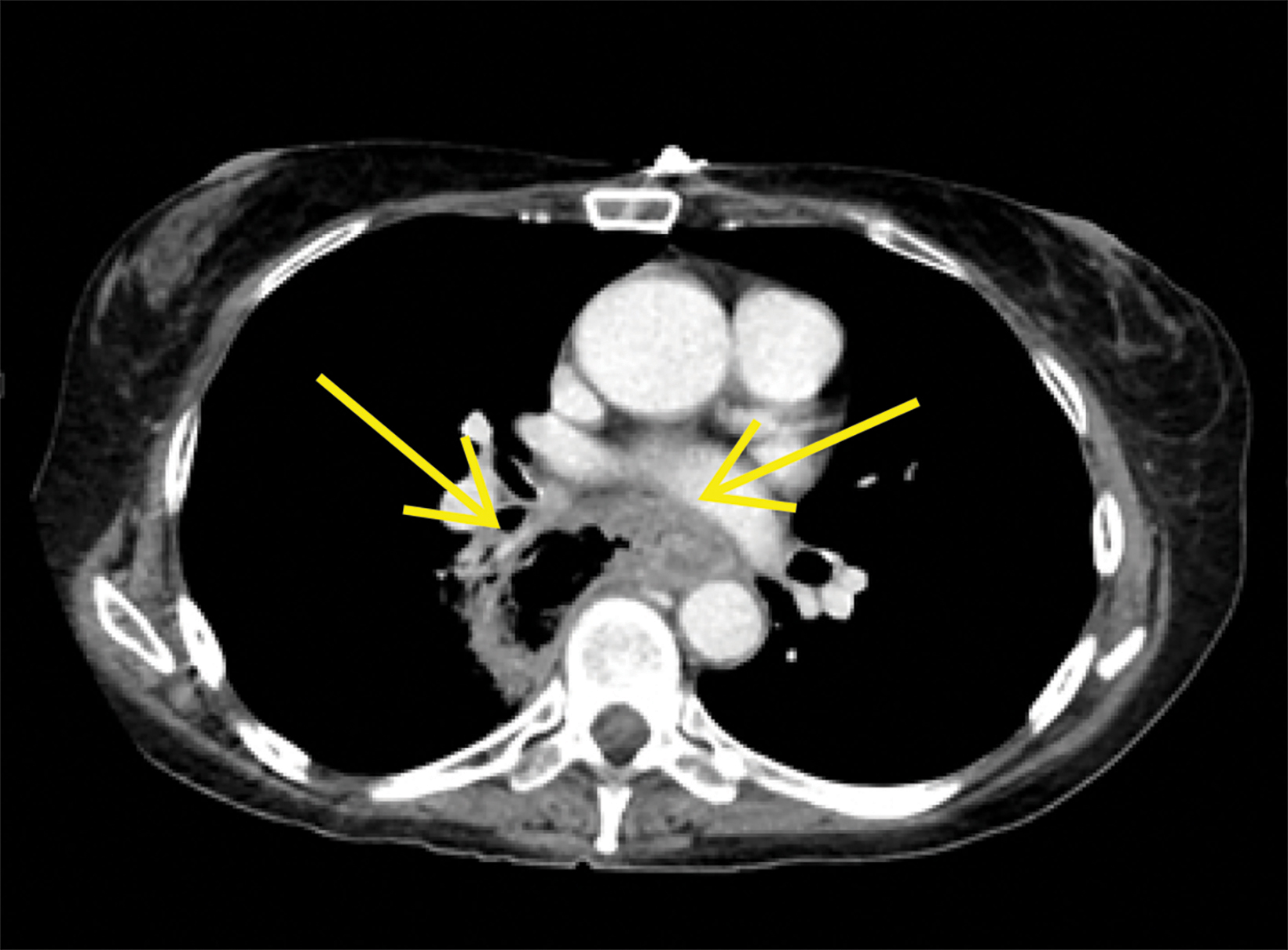
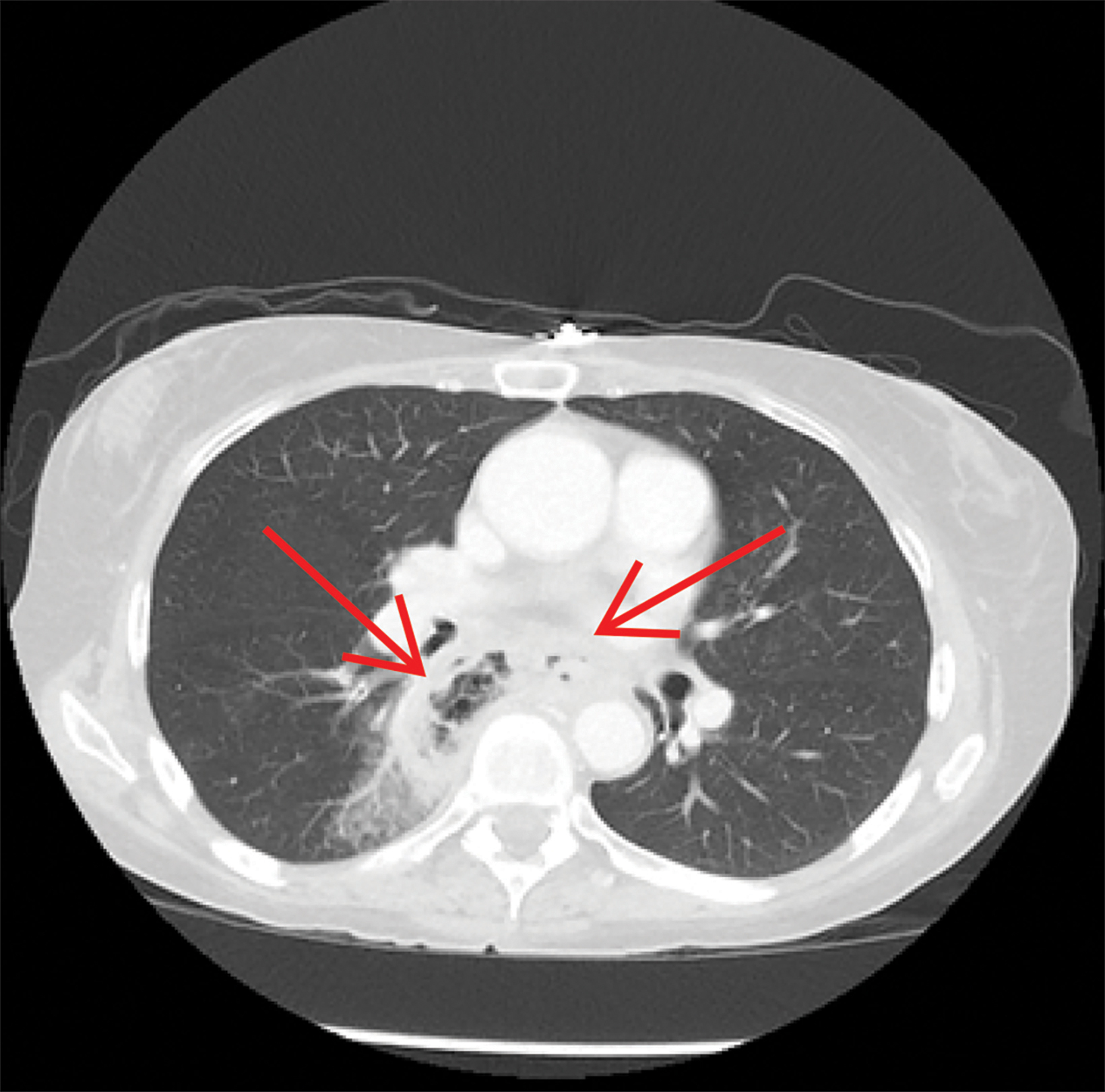
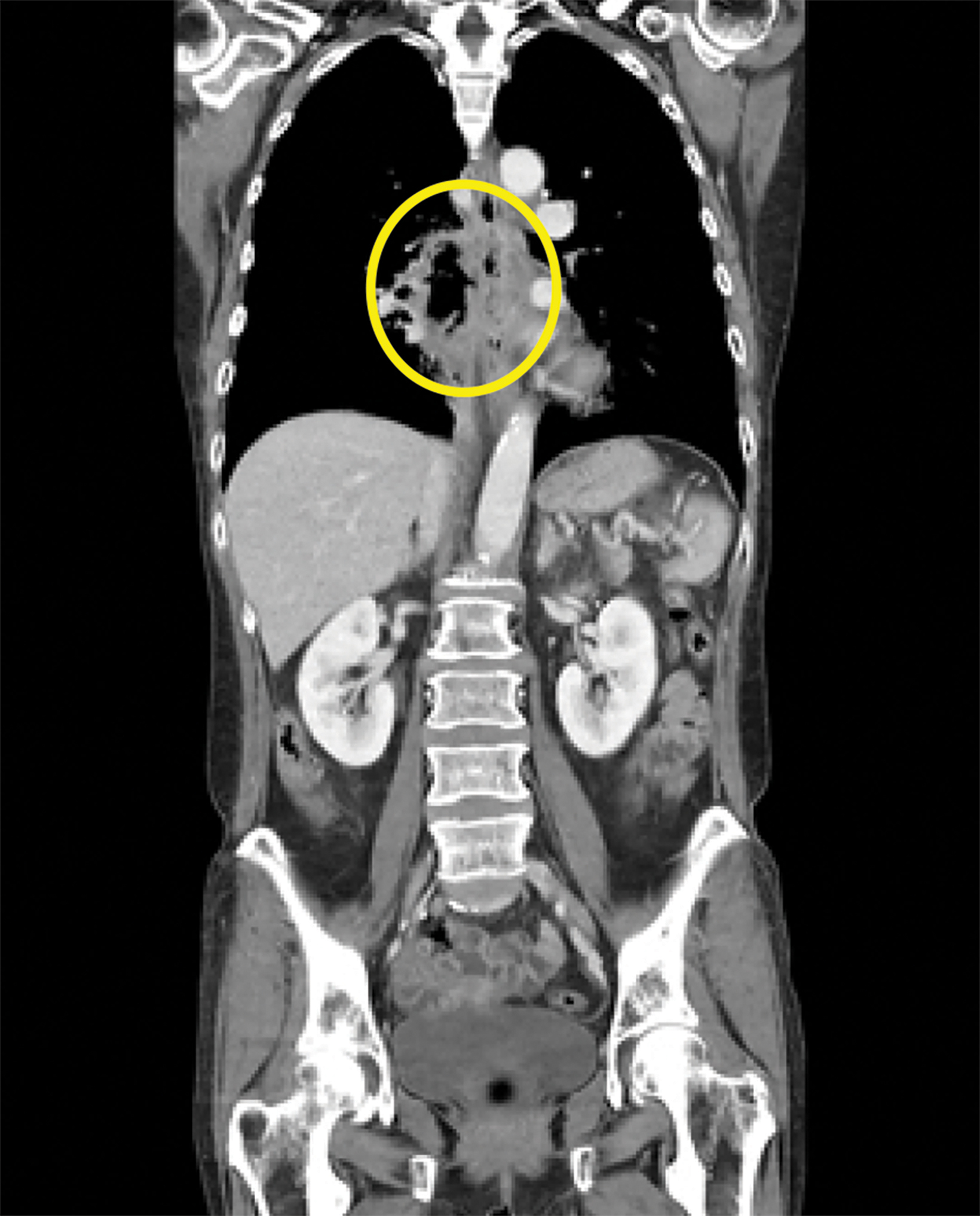
Contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis demonstrated a long segment of circumferential wall thickening measuring 1 cm in the mid-esophagus. A tract was seen extending from the mid-esophagus to a right lower lung cavitation, demonstrating an irregularly thickened wall and measuring 2.5 × 5 × 7 cm (TR × AP × CC). Ground-glass opacities surrounded the right lung cavity (Figure 1).
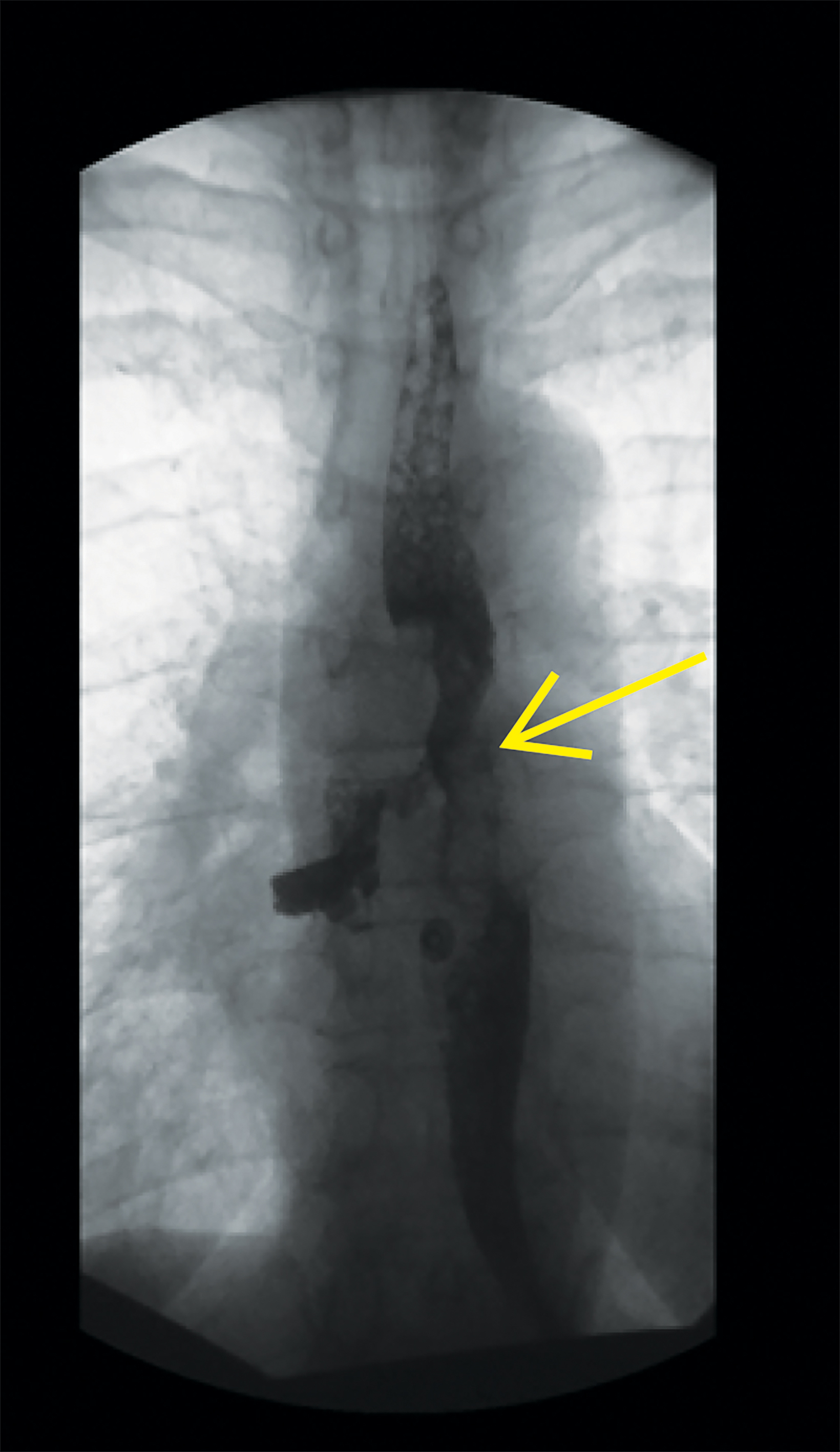
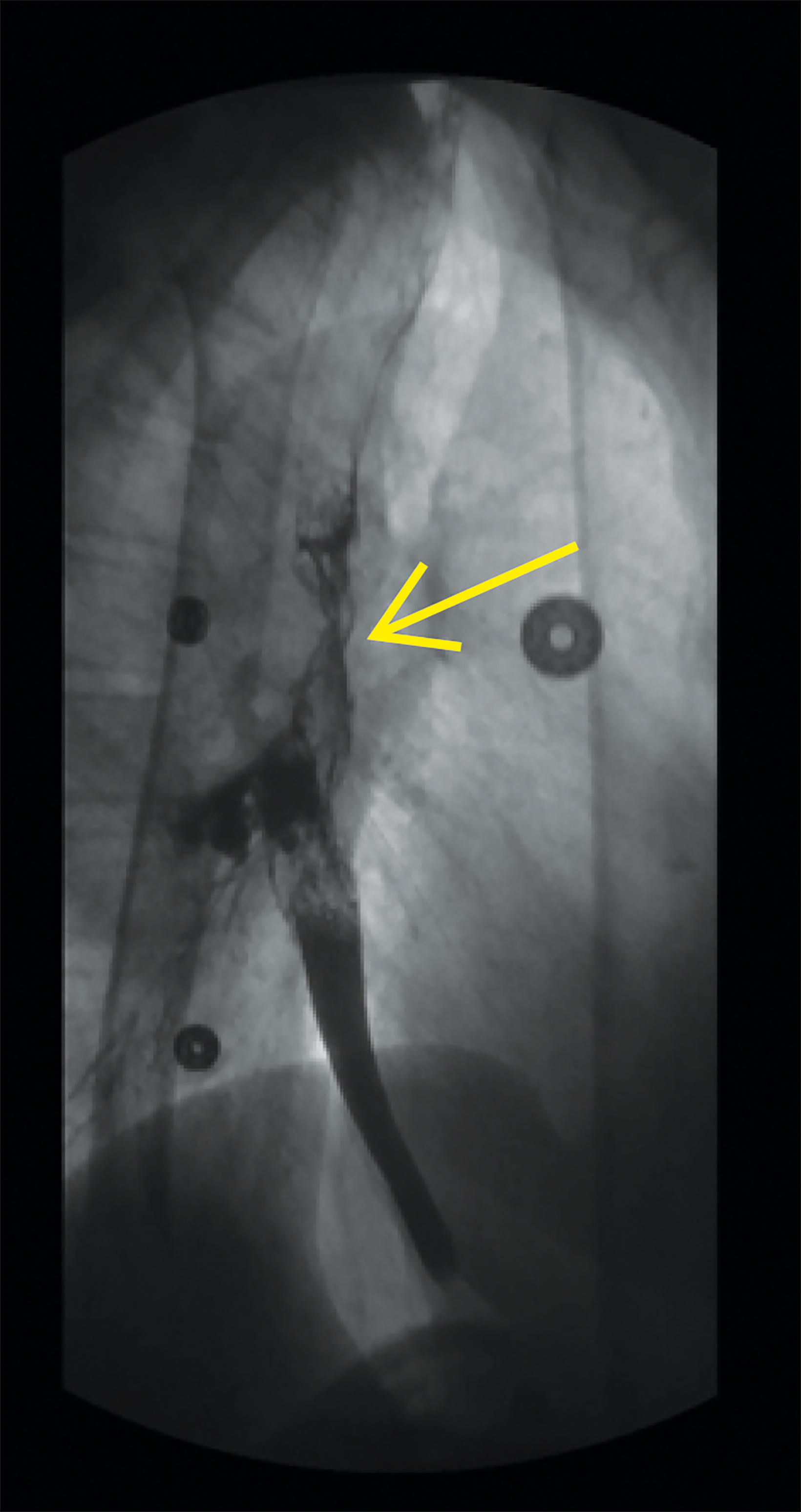
Follow up esophagram with water-soluble contrast demonstrated contour irregularity of the mid-esophagus with contrast extravasation to the right bronchial tree (Figure 2).
Diagnosis
Bronchoesophageal fistula
Discussion
A bronchoesophageal fistula (BEF) is an abnormal connection that forms between the esophagus and the bronchus, most commonly within the right bronchial tree.1 Bronchoesophageal fistulas may be congenital or acquired, with acquired causes being more common.1,2 They include trauma, malignancy, infection, prior thoracic surgeries, silicosis, foreign body ingestion, esophageal diverticulum, or prolonged endotracheal intubation.1,3,4 In a case such as the one described here, a significant history of smoking seemed to be a major contributor to the development of esophageal malignancy. Other risk factors associated with malignancy of the esophagus include hot fluids, alcohol, caustic ingestion, and achalasia.
Congenital causes of BEF are less common and typically appear in the neonatal period, although presentation during adulthood has been reported.5 Congenital BEFs may present with normal mucosal lining within the fistula, unlike the acquired etiologies, which show absence of the normal mucosal lining.6
These entities were initially described in 1965 with a classification system that remains in use today. Type 1 BEFs consist of an esophageal diverticulum forming the fistula, type 2 consist of an extension of the esophagus into a lobar or segmental bronchus (most common), type 3 result from an extending bronchogenic cyst, and type 4 results from a pulmonary sequestration.1,7
Bronchoesophageal fistulas are rare, with very few reported in the medical literature. Diagnosis is commonly delayed or potentially misdiagnosed, whereas tracheoesophageal fistulas, which have a higher incidence and greater association with endotracheal intubation, are typically diagnosed much more quickly.1,6 Delays in diagnosis are usually due to the condition’s nonspecific presen- tation; common signs and symptoms at presentation are chronic cough, hemoptysis, signs of aspiration, dysphagia, abdominal or chest pain, or worsening gastroesophageal reflux.7 Delays in diagnosis are more common among benign causes than malignant cases, owing to the mild nature of pathology in the former. Conventional esophagography is considered the gold standard test for diagnosing BEF, although endoscopy can also be easily used to visualize an esophageal mass.1,3
Bronchoesophageal fistulas are associated with a high morbidity and mortality, and the underlying cause should be investigated. Timely treatment should be initiated to avoid sepsis and aspiration.1,3 Surgical treatment, which consists of excision of the fistula with closure of the abnormal openings, has a high rate of success.1,8 Alternative treatments include either surgical stapling or applying acetic acid and sodium hydroxide to both ends of the fistula.9,10 In cases associated with an underlying malignancy, palliative care may be performed with esophageal stenting.
Conclusion
Acquired BEFs are less common than tracheoesophageal fistulas. While underlying causes vary, malignancy-associated BEFs tend to present with nonspecific, vague symptoms that require further work-up. Once diagnosed, treatment of BEF typically consists of surgical excision unless high-grade malignant cases necessitate palliative care.
References
- Mangi, A., Gaissert, H., Wright, C., et al. Benign broncho-esophageal fistula in the adult. Ann Thorac Surg. 2002; 911-915.
- Kim JH, Park KH, Sung SW, Rho JR. Congenital bronchoesophageal fistulas in adult patients. Ann Thorac Surg. 1995;151–155.
- Lim KH, Lim YC, Liam CK, Wong CM. A 52-year-old woman with recurrent hemoptysis. Chest. 2001;955–957.
- Spalding AR, Burney DP, Richie RE. Acquired benign bronchoesophageal fistulas in the adult. Ann Thorac Surg. 1979; 378–383.
- Iwazawa T, Imazato M, Ohnishi T, Kimura Y, Yano H, Monden T. Double congenital bronchoesophageal fistulae in an adult. Jpn J Thorac Cardiovasc Surg. 2004;52:386–389.
- Aggarwal, D., Mohapatra, P., and Malhotra, B. Acquired bronchoesophageal fistula. Lung India. 2009;261:24-25.
- Braimbridge, M.V. and Keith, H.I. Oesophago-bronchial fistula in the adult. Thorax. 1965; 20:226–233.
- Darbari A, Suryavanshi A, Tandon S, Chandra G, Singh P. Non malignant Tracheo-Esophageal fistula: Our experience. Indian J Thorac Cardiovasc Surg. 2005;21:272–276.
- Smith, B.D. Jr, Mikaelian, D.O., and Cohn, H.E. Congenital broncho-esophageal fistula in the adult. Ann Otol Rhinol Laryngol. 1987;96:65–67.
- Smith, D.C. A congenital broncho-esophageal fistula presenting in adult life without pulmonary infection. Br J Surg. 1970; 57: 398–400.
