Roundtable Recap: The Impact of Neuroimaging in the Clinical Decision Making of Diagnosing Dementia

Editor’s note: Applied Radiology recently hosted a series of four roundtable discussions supported by an educational grant from Biogen in partnership with icometrix and focusing on current and future applications of imaging in dementia management.
This roundtable, the fourth of the series, explored various options of neuroimaging for patients presenting with neurodegeneration, as well as the importance of standardizing protocols in the management of dementia in clinical practice.
The roundtable participants were:
- Suzie Bash, MD, a neuroradiologist and the Medical Director of San Fernando Valley Interventional Radiology at RadNet in Los Angeles, California (moderator);
- Sharon Sha, MD, MS, clinical associate professor of neuroradiology at Stanford Neuroscience Health Center of Stanford University, Stanford, California; and
- Max Wintermark, MD, MAS, MBA, chief and professor of neuroradiology, University Medical Line, Stanford University, Stanford, California.
Dr. Bash began the roundtable with a brief commentary about the prevalence of Alzheimer’s disease—50 million people worldwide are affected—and its growing role as a major health problem. As other neurodegenerative disorders, as well as neurologic conditions like stroke and tumors, can present with similar symptoms, neuroimaging plays a key role in diagnostic accuracy and longitudinal follow-up.
Neuroimaging—which Dr. Sha referred to as the “standard of care” and noted her preference for magnetic resonance imaging (MRI) over computed tomography (CT)—can be used to observe and consider patterns of atrophy in the context of symptoms and overall clinical syndrome. Dr. Sha discussed the value of quantitative imaging in helping to distinguish typical from atypical brain atrophy based on patient age. The standardization achieved by quantitative imaging serves as an adjunct to reduce report bias among different interpreters.
Dr. Wintermark agreed that standardized imaging protocols are important when evaluating dementia because they permit meaningful comparisons and reproducible results. He also said a special type of MRI scanner is not needed to perform “fancy sequences;” a 3D T1 sequence that can be done on almost any MRI machine is all that is required. He also noted that a standardized report and display enables concise and consistent messaging of results to the referring neurologist and the patient.
Dr. Bash added that quantitative tools are particularly useful for longitudinal assessment of disease progression. Even if a prior study is acquired at a different facility, she said, as long as a 3D T1 sequence was performed, both studies can be sent for volumetric post-processing for accurate assessment of brain atrophy over time.
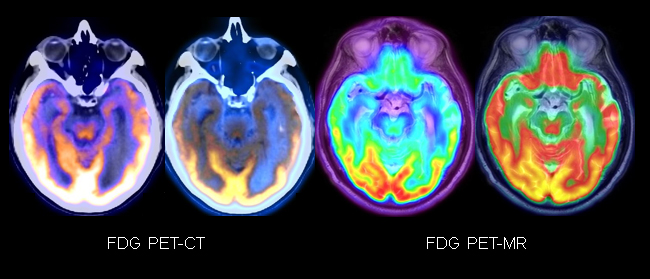
The participants also explored the value of additional imaging techniques, such as brain FDG-PET and amyloid PET imaging, in the setting of neurodegeneration (Figures 1,2).
Dr. Sha noted that the additional imaging information gleaned from these modalities should be considered as part of the overall picture: Do the results fit the patient presentation? Does the quantitative analysis fit, also? How do all these “clues” support the diagnosis?
Since treatment approaches vary, identifying particular dementia subtypes through recognition of specific patterns is important. FDG-PET is particularly helpful, for example, in determining if the patient has frontotemporal dementia (FTD) or Alzheimer’s disease. Tau deposition as seen on PET can also reveal patterns seen on the MRI as cognitive decline accelerates. A stronger diagnosis is made through collaboration, evaluation of atrophy patterns, and assessment of patient demographics.
Dr. Bash agreed that various tools must be used to differentiate Alzheimer’s disease from the normal aging process as well as from other neurodegenerative diseases. “Sometimes it’s very straightforward, but oftentimes, it’s not,” she said.
Advanced neuroimaging techniques are extremely useful, and quantitative imaging is performed quickly and easily. As clinical trials unfold and CMS coverage evolves, the use of these techniques will likely expand and continue to enhance the diagnostic process, the roundtable participants agreed.
Concluding the discussion, Dr. Bash presented many case studies illustrating the impact of neuroimaging in diagnosing dementia, with an emphasis on the impact of quantitative volumetric imaging. She detailed one case that included a bull’s eye graph helpful for differentiating neurodegenerative disorders and presented various imaging examples of the differences between Alzheimer’s disease, Lewy body dementia (LBD), and FTD. Dr. Bash provided evidence that clinical dementia can present with specific imaging patterns on MRI, PET, and quantitative volumetrics, and that imaging plays a vital role in achieving diagnostic accuracy.
