Roundtable Recap: Imaging Biomarkers for the Early Diagnosis of Alzheimer’s Disease
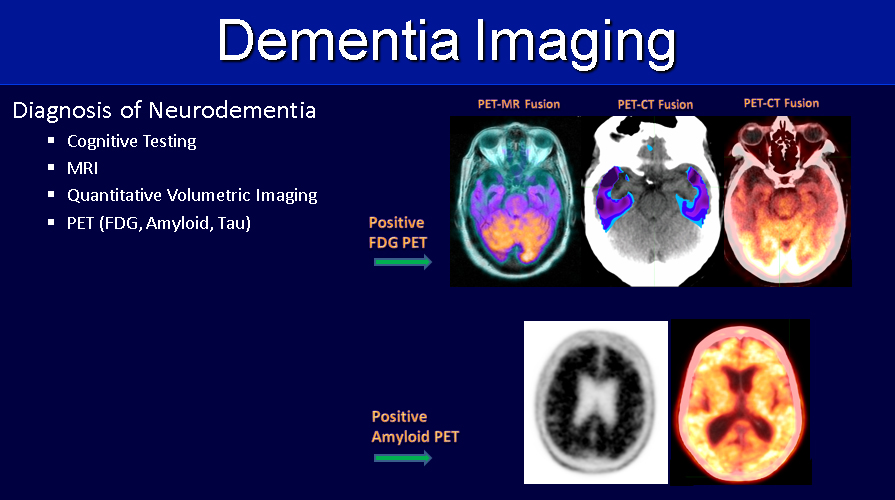
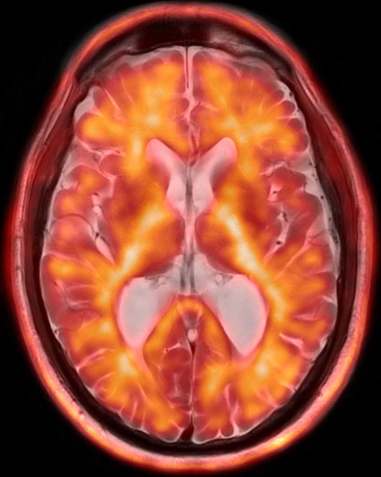
Editor’s note: Applied Radiology recently hosted a series of four roundtable discussions supported by an educational grant from Biogen in partnership with icometrix and focusing on current and future applications of imaging in dementia management.
This roundtable, the third of the series, explored how neuroimaging can assist in the early diagnosis of Alzheimer’s disease. The discussion centered on the NIA-AA classification used for research and how its components apply in the context of the disease’s pathologic changes, imaging’s role in identification and management, and the evolution of neurodegeneration presentation and treatment in the form of amyloid-reducing agents. The roundtable participants were:
- Suzie Bash, MD, a neuroradiologist and the Medical Director of San Fernando Valley Interventional Radiology at RadNet in Los Angeles, California (moderator);
- Sharon Sha, MD, MS, clinical associate professor of neuroradiology at Stanford Neuroscience Health Center of Stanford University, Stanford, California; and
- Max Wintermark, MD, MAS, MBA, chief and professor of neuroradiology, University Medical Line, Stanford University.
Some 6 million Americans have Alzheimer’s disease, which is the sixth-leading cause of mortality in the US and results in the death of about one in three seniors, Dr. Bash said, adding that deaths have increased 145% since 2000.
Recent FDA approval of the first potential disease-modifying therapy (Aducanumab, sold under the brand name AduhelmTM) has made early diagnosis of Alzheimer’s—when it’s most treatable—especially important.
Neuroimaging plays a vital role in assessing many of the biomarkers found in early Alzheimer’s. In 2018, Dr. Bash explained, the National Institute on Aging (NIA) and the Alzheimer's Association shifted the definition of Alzheimer’s disease from the clinical criteria to a biological construct within the research framework, using the A/T/N classification system, where “A” represents the biomarker for amyloid; “T” represents the biomarker for “tau;” and “N” represents neurodegeneration.
The presence of amyloid can be assessed by amyloid PET, cerebral spinal fluid (CSF) via lumbar puncture, or blood; tau, likewise, can be measured with CSF analysis or Tau PET; and neurodegeneration can be measured using brain MRI (sometimes with the adjunct AI-based quantitative volumetric post-processing) with the goal of evaluating the degree of volume loss in brain structures such as the mesial temporal lobes, or with FDG-PET to assess for regions of cortical hypometabolism.
Working with the presence of amyloid and tau biomarkers and evidence of neuronal damage, said Dr. Sha, treating physicians can have greater confidence in their underlying diagnoses. Dr. Bash added that quantitative volumetric imaging is an important tool, as it can be used to calculate the volume of key brain structures and compare it to a normative database to determine the statistical significance of the findings as it relates to neurodegeneration. Quantitative volumetric imaging also enables longitudinal tracking over time, and Dr. Wintermark concurred that these tools have improved significantly over the years, are routinely available, and have great value in managing Alzheimer’s disease treatment.
While amyloid and tau imaging are not currently eligible for reimbursement by the Centers for Medicare and Medicaid Services (CMS), that could change. Radiology is likely to play an increasingly important role in staging Alzheimer’s disease as well as in imaging of the biomarkers, Drs. Sha and Bash noted.
The discussion also addressed the potential impact of increased volume of neuroimaging for biomarkers and the importance of consistent imaging protocols as clinical indicators and biomarkers determine accurate diagnoses.
Of patients who experience mild cognitive impairment, about 40% will progress to Alzheimer’s disease, which Dr. Bash called “a big number,” while emphasizing the importance of neurologist and neuroradiologist collaboration in “really putting the picture together.”
Aduhelm’s hefty price tag makes accurate diagnosis and staging important. There are also critical considerations regarding amyloid-related imaging abnormalities (ARIA)—such as edema or hemorrhage—and the resultant need for safety monitoring via imaging. Of course, physicians must evaluate each patient’s clinical presentation and overall goals for treatment when considering treatment options. Dr. Bash noted that while Aduhelm has demonstrated its ability to remove beta-amyloid plaque from the brain, there isn’t certainty that this translates to improved cognition.
Dr. Wintermark noted that considering the increased number of patients that will require multiple imaging tests over time, imaging sites will have to make accommodations for the expected surge in volume. Radiologists will also have to be educated on ARIA presentation and its implications, and consistent communication between neurologists and neuroradiologists regarding treatment and reporting is a must, Dr. Bash explained.
“We’re really on the cusp of a lot of developments here,” Dr. Sha said, “but I think the exciting thing is having the imaging modalities to help diagnose [Alzheimer’s disease]…and then using those modalities to monitor treatment and making sure they’re safe.”
The discussion concluded with an illustrated summary by Dr. Bash of the role advanced neuroimaging techniques play in assessing pertinent biomarkers for early detection and longitudinal monitoring of Alzheimer’s disease (Figure 1,2).
She also provided an update on the importance of neuroimaging in the wake of recent FDA-accelerated approval of a potential disease-modifying therapy [AduhelmTM], its impact on imaging facilities, as well as the imaging appearance and clinical implications of ARIA.
