AI-Powered Clinical Decision Support Software for Early Lung Cancer Diagnosis Gets FDA Nod
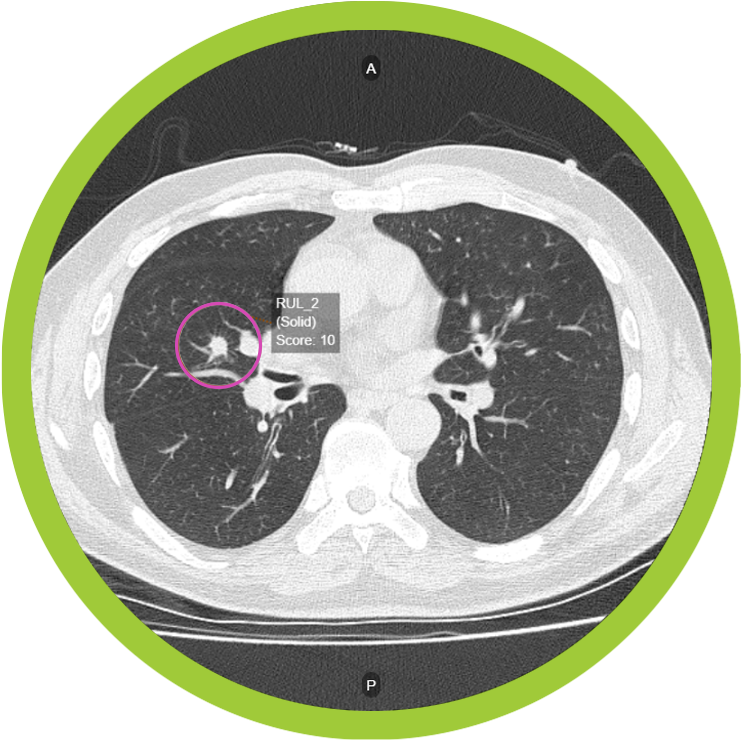 Optellum has received US FDA clearance for its Virtual Nodule Clinic, an AI-powered clinical decision support software for pulmonologists and radiologists managing patients with small lesions, or nodules, in the lungs that could represent early-stage lung cancer. According to the company, this is the first such application of AI decision support for early lung cancer diagnosis cleared by the FDA.
Optellum has received US FDA clearance for its Virtual Nodule Clinic, an AI-powered clinical decision support software for pulmonologists and radiologists managing patients with small lesions, or nodules, in the lungs that could represent early-stage lung cancer. According to the company, this is the first such application of AI decision support for early lung cancer diagnosis cleared by the FDA.
One key opportunity to diagnose more small pre-symptomatic lung cancers earlier is in patients who have a lung nodule identified incidentally during chest CT scans ordered for other reasons, such as emergency room or cardiac scans. Optellum’s Virtual Nodule Clinic enables pulmonologists to identify and track at-risk patients with suspicious lung nodules and make optimal clinical management decisions for those patients. The software features a clinically-validated Lung Cancer Prediction (LCP) score designed to empower clinicians to more accurately and consistently evaluate lung cancer risk and make more optimal clinical decisions. Optellum’s LCP score is powered by the world’s first FDA-cleared imaging AI/”Radiomics”-based digital biomarker for lung cancer. The score is computed from full patterns of 3D pixels in standard images captured by CT scanners.
Physician use of Virtual Nodule Clinic is shown to improve diagnostic accuracy and clinical decision-making. In the clinical study which underpins the FDA clearance, all readers in the study, which included pulmonologists and radiologists of various levels of expertise, from generalists to experts, showed a statistically significant improvement in their accuracy for diagnosing lung nodules when using the Optellum software, with an average improvement of 6.85 Area Under the Curve (AUC) points (p<0.001) and a range of 2.4 to 12.1 AUC points for an individual physician. In this study, AUC measured readers’ average accuracy in correctly classifying nodules as malignant or benign. According to an MRMC study presented to the American Cancer Society National Lung Cancer Roundtable (NLCRT) 2020, use of the Optellum software resulted in improved diagnostic accuracy and more consistency among physicians.
“This study demonstrates the clinical impact of the LCP score,” said Dr. Anil Vachani, Principal Investigator of the study and Associate Professor and Co-Director, Lung Cancer Screening at the University of Pennsylvania. “When using the LCP score, all readers in the study significantly improved both their sensitivity and specificity of diagnosis, and readers at all levels of expertise became more consistent. This is significant because it could assist with early lung cancer diagnosis and intervention in today’s clinical practice, where many patients with cancerous nodules may face delays in diagnosis and treatment, while patients with benign nodules are often unnecessarily exposed to aggressive procedures with sometimes life-threatening complications.”
Optellum’s LCP has been validated in additional multi-center studies and shown to consistently outperform conventional risk prediction models recommended in the current clinical guidelines and considered state-of-the-art in classifying nodules as low (likely benign), intermediate or high risk (likely cancerous). In an independent validation study led by physicians from Vanderbilt and Oxford, the AI was shown to correctly reclassify indeterminate nodules into high- and low-risk categories in more than a third of cancers and benign nodules1, illustrating the potential to speed up lung cancer diagnosis and reduce invasive biopsies and surgeries on patients without lung cancer, compared to the current standard of care.
“We are delighted to launch the world’s first AI-based decision support for early lung cancer diagnosis cleared by the FDA,” said Václav Potěšil, PhD, co-founder and CEO of Optellum. “This clearance will ensure clinicians have the clinical decision support they need to diagnose and treat lung cancer at the earliest possible stage, harnessing the power of physicians and AI working together – to the benefit of patients. Our goal at Optellum is to redefine early diagnosis and treatment of lung cancer, and this FDA clearance is the first step on that journey. We look forward to empowering clinicians in every hospital, from our current customers at academic medical centers to local community hospitals, to offer patients with lung cancer and other deadly lung diseases the most optimal diagnosis and treatment.”
- Massion PP, Antic S, Ather S, et al. Assessing the Accuracy of a Deep Learning Method to Risk Stratify Indeterminate Pulmonary Nodules. Am J Respir Crit Care Med. 2020 Jul 15;202(2):241-249. https://www.atsjournals.org/doi/full/10.1164/rccm.201903-0505OC.
