icometrix Nets FDA Clearance for AI-Based ARIA Diagnosis and Monitoring Solution
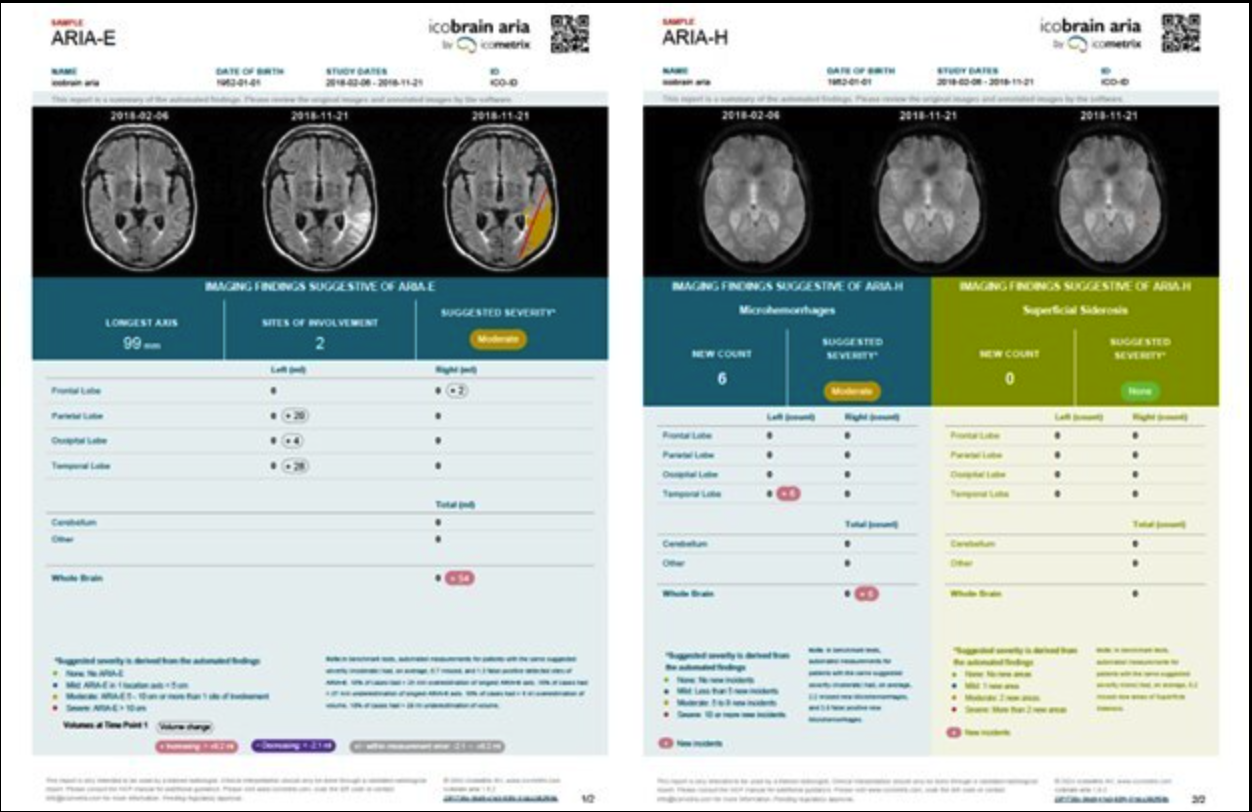
The FDA has granted icometrix clearance for icobrain aria, reportedly the first and only AI software approved for detecting, measuring and grading amyloid-related imaging abnormalities (ARIA), a potentially harmful side effect of new amyloid-targeting therapies. A large study, needed for FDA clearance, demonstrated that the use of icobrain aria significantly increases the accuracy of ARIA assessments by radiologists and hence allows for safer use of new amyloid-beta targeting therapies for Alzheimer disease patients.
“Amyloid-lowering treatments represent an important advance in the treatment of Alzheimer disease, but they are associated with a risk of brain swelling and hemorrhage called ARIA. New standardized tools are needed, such as icobrain aria, to assist radiologists and treating clinicians in detecting and managing ARIA to optimize patient safety,” says Prof Stephen Salloway, Butler Hospital and Professor of Neurology and Psychiatry at the Warren Alpert Medical School of Brown University in Providence, Rhode Island. “I am excited that icobrain aria has received FDA approval, clearing the way for wider use in clinical practice.”
icobrain aria is a unique deep-learning-based AI solution that was trained on thousands of brain MRI scans to accurately detect, diagnose, and monitor ARIA and its severity. The software works in the background and automatically generates an overview report on the ARIA assessment for radiologists using normal clinical MRI scans.
“icobrain aria was thoroughly evaluated in large reader studies. It was demonstrated that the assistance of AI significantly improved radiologists’ ability to detect and classify ARIA, boosting both diagnostic sensitivity and accuracy, and adding a critical safety option for patients undergoing amyloid-targeting therapies,” says Prof Jeffrey Cummings, Director of the Chambers-Grundy Center for Transformative Neuroscience at University of Nevada, Las Vegas.
Alzheimer therapies, including lecanemab (Leqembi) and donanemab (Kisunla), are designed to target amyloid buildup, which is believed to play a role in Alzheimer disease. Together, these treatments represent a shift from symptom management to disease-modifying approaches, offering hope to millions of patients and their families.
However, these promising drugs also carry risks, one of which is the development of ARIA (amyloid-related imaging abnormalities), a complication that can cause lesions in the brain, including edema, swelling and/or hemorrhages. According to the FDA, the prescription of these amyloid-targeting therapies requires intensive ARIA monitoring with repeated magnetic resonance imaging (MRI) brain scans to detect and assess these changes before they pose serious neurological risks. Until now, identifying and evaluating ARIA has been a labor-intensive process, involving detailed, time-consuming, and visual comparisons of the brain MRI scans by radiologists.
“We are thrilled to announce FDA authorization of the first-ever computer-aided detection and diagnosis solution in neuroradiology. The FDA holds devices in this category to exceptionally high standards of evidence and performance, and this approval reflects the strong evidence behind icobrain aria’s ability to help radiologists assess important safety events that can impact treatment and patient care,” says Dr Dirk Smeets, CTO of icometrix.
