Whole-body CT screening
Images



Dr. Horton is an Associate Professor of Radiology at Johns Hopkins Medical Institutions, Baltimore, MD.
Over the last decade, whole-body computed tomographic (CT) screening has become increasingly common. During that time, many have questioned the value of this examination and the effect of profit-making incentives on the selection of CT scanners; the scanning protocol itself, including the choice to use or forego intravenous (IV) contrast material; and the physician-patient relationship.
Incidental findings
For almost 30 years, CT scanning has been used successfully to image symptomatic patients. It is essential to patient care and is heavily relied upon by clinicians and surgeons for the detection and staging of disease, and the monitoring of treatment.
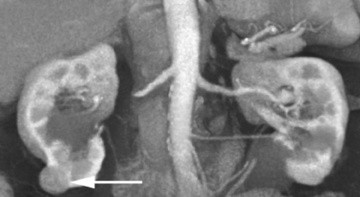
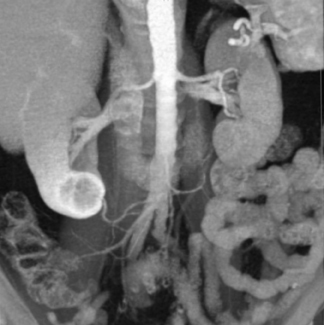
Incidental findings on CT are common. Some are significant, such as aneurysms and neoplasms, and some are insignificant or a normal process of aging, such as renal cysts, calcified granulomata, and arthritis. In our busy department, we detected the following incidental CT findings in just 1 week: adrenal adenoma, lung nodules, renal cell carcinoma in a potential renal donor (Figure 1); carcinoma of the rectum in a patient being evaluated for biliary obstruction; bronchoalveolar carcinoma in a patient undergoing coronary artery screening; and renal artery stenosis in a hypertensive patient being scanned for abdominal pain.
The few studies on CT screening in the scientific literature suggest that perhaps 30% of examinations detect a significant abnormality. One study, from the Mayo Clinic, involved >1500 participants. 1 Investigators detected significant incidental findings in 14%, including abdominal aortic aneurysm, lung cancer, breast cancer, lymphoma, renal cell carcinoma, and gastric cancer. For a screening population of apparently healthy people, that is a high incidence of significant findings.
Brant-Zawadzki 2 reported on the results of CT screening in 1700 people. Thirty-two percent of the studies were abnormal, not including findings of renal cysts or coronary calcification. Among the abnormalities were 19 cancers, 11 aneurysms, 55 gallstones, and 30 ovarian cysts. It is important to note, however, that many of the scans reported in these studies were performed without contrast en-hancement, so it is not surprising that follow-up would be necessary to clarify some of the abnormalities.
Driving forces
Clearly, we can use CT to screen for and detect disease in asymptomatic people. The controversy lies in whether, as radiologists, we should be offering this service.
Radiologic screening is not a new concept. We have been performing barium enemas, chest X-rays, mammograms, and dual-energy X-ray absorptiometry (DEXA) for years. It is not difficult to justify using CT to screen for lung cancer in smokers or to perform virtual colon-oscopy in patients over the age of 50 who are at high risk for colon cancer. What is new is the use of whole-body CT to screen for unspecified disease in people who are not at high risk.
Whole-body CT screening gained national attention several years ago when Oprah Winfrey had a full-body scan and reviewed the results with a radiologist on her television show. Demand for whole-body screening has risen sharply since, and whole-body imaging centers have opened throughout the country.
Improvements in CT technology have been a driving force in whole-body screening. Until the mid-1990s, CT scanners were not capable of detecting a 5-mm renal cell carcinoma, or a 1-cm pancreatic neoplasm. With the introduction of multidetector CT (MDCT) technology, it became technically feasible to screen asymptomatic people. Faster scanning meant less motion artifact and, together with thinner collimation (0.75 mm or 0.6 mm) and isotropic data sets, created high-resolution, high-quality images.
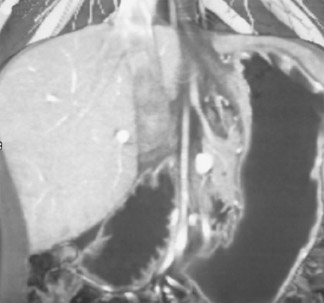
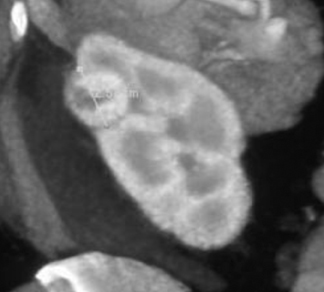
Figure 2 shows a very small hepatoma in a cirrhotic liver. Without MDCT technology, it would not have been possible to scan fast enough or acquire data in thin enough slices to identify such a finding. Small lung nodules, carcinoid tumors, and renal artery aneurysms are other examples of subtle findings that are more easily seen on high-quality 3-dimensional (3D) images.
Another reason whole-body CT screening became popular in the mid-1990s was increased awareness of screening tests in general. People were already knowledgeable about the importance of screening for high blood cholesterol, breast cancer, lung cancer, and prostate cancer, among other conditions. They viewed CT simply as a new screening modality, not as a new concept.
People were also more health-conscious and, in part as a reaction to changes in health insurance, felt the need to be more in control of their health-care decisions. They considered CT screening a source of additional information to use in monitoring their own health. Under these circumstances, it's not surprising that physicians teamed up with entrepreneurs to open whole-body CT screening centers.
Imaging protocol
At many screening centers, whole-body CT scans are noncontrast studies of the chest, abdomen, and pelvis. The quality of the equipment and the experience of the radiologist vary from one screening center to another. The CT scanner may be electron-beam, single-detector, or multidetector. There is no quality control; any type of CT scanner can be used for screening studies.
The technique also varies widely from center to center, based on the type of scanner. Slice width can range from 1 to 10 mm. Usually, the study includes some type of 3D image reconstruction. The examination may or may not require a doctor's referral. The study usually also includes a consultation with a radiologist or a clinician, such as a nurse practitioner or internal medicine specialist, to explain the findings to the patient. In some cases, the whole-body scan is combined with other types of screening studies, such as a heart scan or virtual colonoscopy.
If CT screening is to be done appropriately, it is necessary to determine the best protocol and approach to screening healthy people. The top 2 causes of death in the United States are cardiovascular disease and cancer; therefore, the screening protocol should be designed with that in mind.
At Johns Hopkins Medical Institutions, we use our best scanner, a 64-slice Siemens Sensation (Siemens Medical Solutions USA, Malvern, PA), and we always do 3D imaging. We start with coronary calcium scoring and then scan the chest, abdomen, and pelvis, using IV contrast unless it is contraindicated or the patient declines its use after weighing the risks and benefits. We always give the patient the option of meeting with us afterward to review the findings.
Table 1 outlines the whole-body imaging protocol we use at Johns Hopkins. It is, essentially, the same protocol we use for CT angiography. Note that we use 0.75-mm collimation and reconstruct at 0.5-mm intervals. We maximize our protocol to detect cardiovascular disease and small tumors.
We instruct patients to drink 750 mL of water as oral contrast. This avoids interference of high-density contrast material with 3D reconstructions. For contrast-enhanced examinations, we use nonionic isosmolar contrast material (iodixanol, 320 mgI/mL), intravenously injecting 120 mL at 3 to 5 mL/sec. We initiate scanning 3 to 60 seconds after beginning the contrast injection. Some radiologists like to scan very early to focus on the arterial phase; others prefer to wait, so that kidney enhancement is optimized for the detection of small renal-cell carcinoma. We scan from the base of the skull through the pubic symphysis.
We always use iodixanol for screening studies because of its safety profile. People who undergo whole-body CT screening are generally healthy, and we want to minimize the risk of a serious contrast reaction. With an osmolality of 290 mOsm/kg, iodixanol is isotonic to human blood and, therefore, is better tolerated and less likely to cause cardiac or renal toxicity. (Unless the patient reports a history of kidney disease, we do not check the pa-tients' creatinine levels prior to CT scanning, as we use iodixanol in all cases).
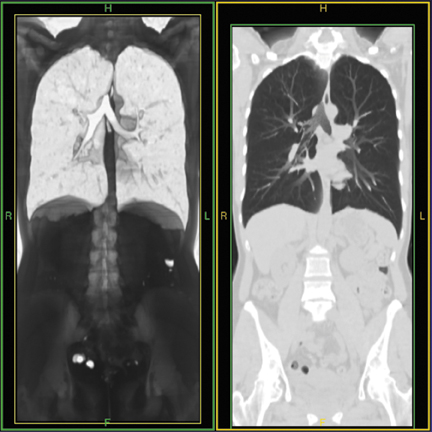
Another advantage of using isosmolar contrast material is that it appears to mix better with blood, resulting in more uniform opacification. Figure 3A shows a CT scan for suspected pulmonary embolism (PE). Low density in the center of the pulmonary artery results from unopacified blood. By comparison, as shown in Figure 3B, the use of isosmolar contrast material enables detection of a small, discrete filling defect within the pulmonary artery, as would be expected with PE.
Image review
Three-dimensional imaging plays an important role in CT screening. It is much easier and faster to evaluate a large volume of information using 3D reconstructions, rather than by reviewing 2000 axial slices. Also, 3D reconstructions are useful when discussing scan findings with a patient.
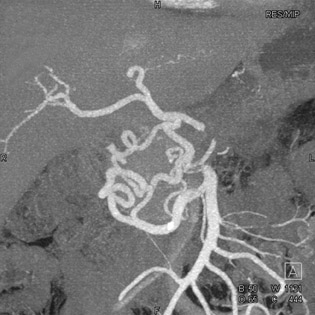
Usually, I have the patient sit with me at the monitor as I reconstruct the 3D study. The consultation typically takes approximately 10 minutes. As shown in Figure 4, I point out the lungs; major abdominal organs, including the kidneys, liver, and spleen; and the bones. I also spend a fair amount of time reviewing angiographic images, looking at the abdominal and thoracic aorta, mesenteric vessels, and renal arteries.
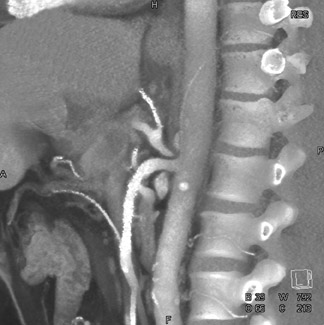
Since we begin image acquisition at the base of the skull and do a relatively early-phase contrast injection, we can also review the carotid arteries for atherosclerotic disease. Figure 5 shows a patient with extensive atherosclerotic disease in the carotid arteries.
Contrast debate
The use of IV contrast media in whole-body CT screening is controversial. Arguments against this practice include the risk it poses to a healthy person of having a serious contrast reaction, the potential for nephrotoxicity, the added cost of the contrast agent, and the necessity of having a physician on site to treat any contrast reactions. In reality, most centers, as profit-driven businesses, focus on the latter two reasons in arriving at the decision to forego contrast-enhanced studies.
A key advantage of using IV contrast is that the resulting scan is more sensitive and much more specific for the detection of small lesions, and for characterization of any abnormalities. If a noncontrast study reveals an abnormality that requires further characterization, as is often the case, the radiologist will recommend a second, contrast-enhanced study. As a result, a healthy person must undergo 2 CT scans instead of 1.
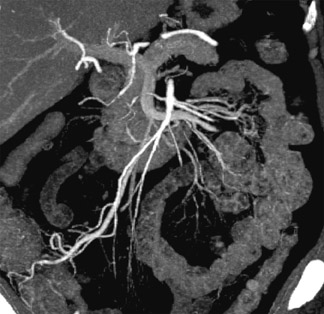
To optimize disease detection in a healthy screening population, we must be able to find subtle tumors. To look for vascular disease, we need to examine the carotid arteries, the aorta, the mesenteric arteries, the renal arteries, and other vessels. Once we find a lesion, we need to be able to characterize it without recommending unnecessary follow-up studies.
Figure 6 illustrates small tumors that would not be visible on a noncontrast study. Noncontrast studies can detect large tumors but offer little value for screening, as patients with large tumors would undoubtedly be symptomatic.
Radiation exposure
Another controversy is whether we should be exposing healthy people to radiation. McCauley 3 estimated that screening CT would induce more cancers than would be cured by early detection, particularly in people who underwent annual screening.
Brenner and Elliston 4 also estimated the radiation risks associated with full-body CT screening. These investigators estimated that a single full-body CT scan in a 45-year-old would result in an increased lifetime mortality risk of approximately 0.8%, mostly from lung cancer. An annual full-body CT scan over 30 years would increase the incremental risk to 1.9%.
Realistically, any increase in risk is probably minimal. Although some centers recommend yearly CT screening, it's unlikely that many people would undergo annual CT scans. (We recommend that patients repeat CT screening in 5 to 10 years, if at all.)
Nonetheless, it is important to keep radiation dose in mind and take steps to minimize it. New scanners with dose-modulation capabilities reduce radiation exposure. Another key step is optimization of imaging protocols to minimize radiation dose, taking into account body size, for example. It is also important to establish age guidelines. In my opinion, patients should not be screened before age 40.
Value
In September 2002, the American College of Radiology issued a statement indicating there was insufficient scientific evidence to justify recommending total-body CT screening. 5 It is unlikely, however, that randomized, prospective studies will ever be done to assess the value of CT screening, as it would take millions of people and decades of follow-up to determine whether CT screening influences the natural history of disease or mortality.
The absence of proven value is not unusual in medicine, however. Many of the examinations and procedures we do every day have not been subjected to rigorous scientific evaluation. The impracticality of conducting a large, randomized, double-blinded trial of CT screening should not necessarily dissuade us from pursuing CT screening.
Beinfeld et al 6 recently estimated the cost-effectiveness of one-time whole-body CT screening from a societal perspective. They assumed that any benefits from screening when compared with routine care were due to earlier detection of disease and improved survival. Estimated costs, including the screening exam, follow-up tests, and patient care, totaled $2,513 per patient, or $151,000 per life-year gained. This analysis suggests that CT screening is not a very cost-effective approach to population screening.
On an individual basis, CT screening can have positive health effects. Some people, upon receiving a clean bill of health, are inspired to make healthy lifestyle changes, including smoking cessation. However, screening can also have negative effects. Ostroff et al 7 suggested that individuals with a normal CT scan were less likely to quit smoking.
I always advise patients that CT does not evaluate organ function, and that a person can have a normal CT scan and still have significant disease. With proper counsel, most patients appreciate the limitations of CT and understand that it does not replace routine medical care or justify taking unnecessary health risks.
Self-referral
One area of controversy that must be addressed is the need for physician-referral. Some self-referral imaging centers essentially give the results to the patient and send them out the door. This is a risky practice for both patient and radiologist. A radiologist who performs a screening study without a physician referral establishes a patient-physician relationship and assumes the responsibility for follow-up.
At Johns Hopkins, we require a physician referral. We discuss the findings with the patient, but we also send the results to a physician who can further explain the findings, if needed, and ensure that the patient receives necessary follow-up.
The number of self-referral body imaging (SRBI) centers is growing. Based on a series of Internet searches, Kalish et al 8 reported that the number of SRBI centers totaled 161 in 2003, up from 88 in 2001. Of the centers accepting self-referred patients, 66% were solely SRBIs, 17% were general diagnostic imaging centers, 11% were hospitals, and 6% were compre-hensive screening centers. Of the SRBIs, 94% performed heart scans, 84% full-body scans, 78% lung scans, 55% virtual colonoscopy, 20% abdominal-pelvic scans, and 16% head scans.
The largest number of self-referral CT screening centers, nearly 40%, were in the western United States, with another ap-proximately 25% in the South. Centers tended to be located in areas with higher per-capita and median household incomes and higher percentages of people with college and advanced degrees.
Is whole-body CT screening good medicine or simply greed? Medicare and other types of health insurance do not cover these studies. Instead, patients pay cash, making this a potentially lucrative for-profit venture. Body imaging centers send coupons in the mail, conduct aggressive advertising campaigns, and set up business in malls, all to encourage people to undergo CT screening. It is not uncommon for SRBIs to spend a great deal of money on decorating while using a mediocre CT scanner to perform the screening examination and send studies off-site for interpretation at the cheapest rates.
There are signs that the market is becoming saturated, however. Competition between body imaging centers is increasing, and the charge for a CT screening scan is falling, in some case to one third to one half of the initial $800 to $1,000 price tag. There are even mobile scanning companies that charge as little as $75 per body part scanned.
How to succeed
To be truly successful at whole-body CT screening-defined by finding very early-stage lesions, not simply by making money-it is necessary to invest in the latest CT technology. Imaging protocols must be designed to maximize the detection of important pathology. It is important to use IV contrast and perform 3D and angiographic imaging. Radiologists must be experienced at discriminating meaningful and incidental findings, report them decisively and clearly to clinicians, and have a good rapport with patients. The approach to image interpretation must be very organized. It is particularly important that follow-up recommendations for incidental findings be uniform from one radiologist to another.
It is also important to be honest and careful about making claims. One CT screening center claimed on its Web site that the radiation dose from a CT scan is equivalent to that of sitting in the sun for 15 minutes. Making such laughably false claims is a serious breach of ethics. It is important to acknowledge, in terms that patients can understand, that CT scans involve radiation exposure. Only then can they can make an informed decision whether or not to take that risk.
Another center claimed that anyone over the age of 30 with a family history of heart disease, cancer, diabetes, high blood pressure, high cholesterol, or chest pain, or a personal history of smoking or a sedentary lifestyle could benefit from a CT screening examination. It is inappropriate to use such claims to entice younger people to undergo CT screening.
Another center claimed that full-body scanning could detect heart disease when the coronary arteries are only 5% blocked. This statement is clearly misleading. It may be possible to see a tiny calcification at this stage, but it would be clinically insignificant for the patient.
One of the most outlandish claims stated: "Full-body scanning has been certified by the FDA to detect lung cancer and frequently detects incidental tumors, cancers, and other disorders years in advance of symptoms." This makes it sound as if the Food and Drug Administration has approved whole-body CT screening, which is not the case. The claim continued: "Body scans can detect: heart disease, thyroid disease, ovarian cancer, breast cancer, prostate cancer, etc."-essentially, any and all diseases. This type of creative advertising by profit-driven entrepreneurs gives CT screening a bad name.
Conclusion
CT screening presents both significant challenges and significant opportunities for radiologists. Lung-cancer screening and CT colonography are becoming increasingly common, and people continue to want whole-body scanning, despite the controversy associated with this study. Radiologists who include CT screening in their practice should do it well:
- Invest in technology.
- Incorporate IV contrast.
- Require a physician referral.
- Develop a good rapport with the patients.
- Be honest about the strengths, limitations, risks, and benefits of whole-body CT screening.
Discussion
ELLIOT K. FISHMAN, MD: Karen, at Hopkins, where are most of the people who are getting whole-body screening CT coming from these days?
KAREN M. HORTON, MD: We have a group comes from Executive Health who sends executives, since they push screening and preventive medicine. The women will come for mammograms; they'll have their Pap smears and will be evaluated by a cardiologist. In the past, they would do a chest X-ray. But now they offer these people whole-body screening as one extra check. In a way, it's almost like another lab test or another physical examination. So, in addition to going to see the physician, doing a physical examination, which isn't that sensitive, you can do a CT scan, and you're little bit more sensitive in that group of patients.
Then we have some other clinicians who are very proactive for preventive health. They mention to the patients that they might want to get their coronary screened. If they want to get their coronary screened, they ask if they also want to get a full-body screening.
We don't do self-referrals, and we don't advertise the screening or try to encourage people to come in for it. But we do offer it.
FISHMAN: Do you offer screening at Stanford?
GEOFFREY D. RUBIN, MD: We do not do whole-body CT screening at Stanford, and we very rarely do lung cancer screening studies. We do coronary calcium screening studies, and virtual colonoscopies, and that's it with the CT scanner.
FISHMAN: I guess the interesting thing is when you break the things apart. We don't do lung cancer screening, typically, either, except as part of the National Lung Cancer Screening. We're also doing some minority screening of HIV patients in terms of increased lung cancer. It's a project that Lilly's just starting. But again, those are high-risk groups. Of course, the data is going to come along, as you are well aware.
I have no doubt that, in the long term, coronary screening is going to be a routine study. With regard to CT colonoscopy, the results of Perry Pickhardt's seem to be very, very strong. It's almost to the point that if you do the coronaries and you do the colon, in a sense, you are really screening the whole chest and abdomen, basically, if you were doing just those two components, right? If virtual colonoscopy became a mainstream study and coronary screening became a mainstream study, you might get both of those done at age 50. If you did the colon, you essentially get the whole abdomen for free.
RUBIN: I disagree, because there are two big differences. Number one, at least at this stage, to do the colon, you don't need to give intravenous contrast. Number two, to sufficiently study the colon, you can use a much lower radiation dose than you would to get a diagnostic study of the solid organs of the abdomen. So I think it's a pretty big jump to get a diagnostic study for finding renal cell carcinomas, or small hepatic lesions, and such from a colon-screening study.
FISHMAN: What do you think?
STANLEY GOLDFARB, MD: Well, let's use the examples of screening PSAs or mammography. Could we have anything more controversial than identifying a population of patients that really benefits from either of these tests? Some high-risk patients have been identified in whom these tests are quite useful. But for PSAs, for example, one could argue endlessly about whether we are helping patients by taking out these localized prostate cancers that were first identified by PSA. Only about 4% of men die of prostate cancer, but 80% of men have prostate cancer. So I think the problem is in identifying who benefits from a screening test.
You pointed out that under ideal circumstances, as an expert radiologist, you could look at these and make a really informed decision about whether or not you should pursue an abnormality you have identified. But that expertise may not be available at every center, in every region in the country. So, therefore, you're going to be left with harmless abnormalities that lead to surgery or biopsy, and every one of those procedures is going to have an associated morbidity and mortality with it. If you have a disease that occurs in 1% of the population, and your test has a 1% false-positive rate, every time you identify an abnormality, 50% of the time it will represent a false-positive result. The key is to study patients with a high pretest probability of the disease so that a positive result is likely a true positive.
I personally would be happy to go to Johns Hopkins and have this screening done because I am sure you have the expertise. On the other hand, I really should not have the test done anywhere.
FISHMAN: It's an interesting thing. It's like many things, looking at what's good in a selfish way. There are two different things: What's good for society, and what's good for you, the individual. So, as a society, there's no doubt, that whole-body screening would be an impossible thing to do, unless we had incredible computer-aided detection and the cost of studies went down, etc.
But for an individual patient, you can think about it this way. The second you're born, the only sure thing is that you're going to die; the only thing we're really arguing about is when. We have no control in some sense; genetics plays a major role in how long you live. Then the question is what you can do to increase your odds of picking up things that can be addressed, such as renal cancers.
There were a number of articles written in the past couple of years about aortic aneurysms. The Wall Street Journal had a very good meta-analysis quoting the then Journal of Surgery that 15,000 people die each year of ruptured aortic aneurysms. All of these patients could be screened, and 95% of the aneurysms could be salvaged, because they'd be detected early. That's a true number of patients.
I agree that screening is a difficult thing to grasp, because all you have to do is look, as you said, at mammography. Mammography has been around forever. Every couple of years, the question arises again: Is mammography screening worthwhile or not? As you mentioned, PSA screening is a mainstream of modern medicine; but is it worthwhile or not?
With whole-body CR screening, I think the question is whether or not the fact we have the capability means that we should use it. It's an argumentative question, really, because you're not going to come to an answer. As Karen said, when we screen at Hopkins, we use isosmolar contrast to make it the safest thing possible. Do you have patients ask you about that?
GOLDFARB: Yes. They ask, and I tell them what I would do. I think that this is a problem of being able to understand probabilities. It is a question you tend to ask as an individual, and then you come to the wrong answer as an individual, because this is an intuitive, rather than a rigorous mathematical, assessment. I am probably as guilty as anybody of reacting intuitively to data. But the truth is that if you have a finding on a screening with a 1% false-positive rate, a pretest probability of 1% that you have the illness, and say, a 10% complication of the therapy, and, it is counterproductive to go ahead and pursue this test, because you're much more likely to hurt yourself than you are to suffer from the disease. So having said that, you then study specific illnesses, where you start to develop the data that say it is a correct thing to do. For example, for aortic aneurysms, if it is a specific diameter, we know the probability that something of that size rupturing and, therefore, you inform the patient that he has, say, a 25% chance of dying in the next few years. If there is a 2% mortality of the surgery, the odds are in his favor to pursue the surgery. But when it's a 1% chance of rupturing, and a 2% percent chance of dying in the surgery, it's not in his best interest to go ahead and intervene at that point.
We need to develop such detailed information about each disease that we might uncover in a screening test before we can advise patients to help them make an informed decision about whether the screening procedure makes sense.
JULIA FIELDING, MD: We were talking earlier about how the public has changed and wants more knowledge and more control over healthcare. My problem initially with whole-body screening was that it was economically driven. It was never driven from science; it was driven from profit. That's how it started.
I thought it was a bad concept because it started for profit. So I was worried about it that way. Then, of course, the issue of screening healthy people is a big problem, too, since there is no outcome data about whether or you're helping or hurting them. I think that was why it became so contentious among our group, because we had problems both on the economic front, and then also the chance that we were going to hurt somebody.
HORTON: It's interesting, too, because, in this case, the patients are in control because they can pay their money and get it. Up to this point, tests were what their doctor recommended: If you needed a mammogram, the insurance paid. I remember talking to a group of cardiologists when they first started doing coronary screening, and half of them were for it, and half of them were against it. They were arguing whether or not it was going to save lives. They were arguing whether we should do it on the population basis, but they all had theirs done because they wanted to know about themselves.
GOLDFARB: But that's my point. It's the rational behavior. Even though it's done. Don't confuse what the physician does as necessarily being rational.
I understand the dilemma here, but we can argue about opinions. We should not be arguing about the facts. The facts are that we don't know the outcome of some of these studies. For other studies, however, we do know the outcome of screening. Screening for colon cancer is incredibly sensible, and I get my colonoscopy as frequently as my doctor tells me to.
FIELDING: You're the only person in America who does.
GOLDFARB: On PSA, I had a long discussion with my own physician on the rationale for the test in my own case. I acceded to his request but I do not personally think it was all that rational.
FIELDING: Well, PSA has become a cottage industry.
GOLDFARB: There is going to be a refinement of the test that will allow it to be very, very helpful, as testing is for aortic aneurysms. I think the question isn't so much whether you should screen people; it's what should you screen them for and which people should you screen. Unless you can answer that question with some reliable data, you may be doing more harm than good.
FISHMAN: Yes. With whole-body screening, it's interesting. The people who went in it for the money are out of business, because whole-body screening was much more logical at NASDAQ 5000, but now it's at 1500. So they're gone. The fact that some people said that 90% of the studies were positive, that 80% or 90% of all normal people are abnormal, is beyond reason. No, we don't spend 2 hours with every patient.
The way we thought about it was to think about it as a lab test. But instead of the lab test that's specific for one thing, it's like when you go to a doctor at age 50 and they order a full laboratory screening--they check every one of those boxes from PSAs on down.
So think about whole-body screening in the same ways. You get the coronaries and the whole vascular system, so cardiovascular disease is the number-one thing to look for. You get the aorta and get all of these different pieces. So if you really want to find what the value is, you'd have the value after each one of those things. Aortic aneurysm, what's the risk of that? Kidneys, what's the risk of having a renal-cell carcinoma? Again, although 70% of renal cell carcinomas are picked up by serendipity, on any 1 patient, to find one is going to be an incredibly small chance. We're not looking for pancreatic cancers; but you can find them and we all do. We've found a number of nonfunctioning islet cell tumors. But again, you would almost need to say that too with every single potential risk factor, if you did everything. As Karen said about reading the studies, we all know colleagues, particularly residents and fellows, who cannot read a CT scan as normal.
Another big thing in terms of having experience is, when you're 50 years old, unfortunately, you don't look the same as when you were 20. So there's a normal CT scan for a 50-year-old, or a 60-year-old, and a normal scan for a 20-year-old. You just can't be reading the scans as if it were age blind. So I think it will be interesting to see what happens. Some centers do a lot of whole-body screenings. The Mayo Clinic has a large screening program. They do a lot of executive health and have very, very strong patients who demand them. The original demand at Hopkins was from Executive Health.
FIELDING: But this is another problem I had with this. I'm a dreamer or an idealist, I guess. I had to ask if this was all about cash on the barrelhead. It is not covered by any insurance, so you have to have the money to pay for it. So you're automatically stratifying the group because people who can pay are allowed to have this technology. The people who can't pay are not allowed to have this technology. I had a problem with that from the get-go in a state hospital.
At some point, and it's not my decision to make, that's going to be a societal thing. It should also be determined whether or not the test is beneficial to any particular group. But, right from the beginning, you're saying, "I'm withholding this from you." I wasn't ready to do that. To be honest with you, I'm in a state hospital. I don't have a boutique practice. I have indigent care. I've got people, illegal aliens, with active tuberculosis. I get all that kind of stuff coming across the border all the time. So I don't think it would be viable for us to do that, because we have 4 people who would come for it.
RUBIN: Well, we certainly don't live in a socialist country; we live in a capitalist one. So I don't reject outright the notion that some people can afford to pay for things that other people can't.
FIELDING: But we haven't made that decision in medicine yet.
RUBIN: I understand. But I wanted to get back to some of the comments that Elliot was making. I still have concerns about this whole process, mostly from the treatment end. For many cancers, you know what they're going to do--they're going to grow, they're going to get to advanced stage, and you need to take them out. Now, you can make arguments about prostate cancer and renal-cell carcinoma as to whether or not they're actually going to kill the patient, but colon cancer, like many other cancers that we detect, is fatal if untreated.
But for the biggest killer of Americans, cardiovascular disease, we really don't know what to do with the information from a screening study. Even if we just take it down to the coronary calcium question, experts suggest that the identification of coronary calcium should allow for patients to be more aggressive in modifying their risk factors. But, to date, I'm not aware of any data to indicate that increasing statin therapy in patients with high coronary calcium has resulted in a reduction in adverse outcomes in those patients. Even though we can identify potential algorithms, I don't think any of them have proven yet to be effective.
If we take it to the next level of giving contrast medium and looking for carotid stenoses or even coronary stenoses through coronary CTA screening, the questions become even greater with more uncertain outcomes. If we detect a 50% stenosis or a 30% stenosis in the LAD of a patient, does that patient need to go to the cath lab to be stented? What is it that we need to do? I think that it's a big challenge. It's easy to look, but then you have to live with whatever decision you're going to make, and we just don't have any basis to make those decisions right now.
FISHMAN: Geoff, I agree, I think it's a very controversial area and that it's an area you're just never really going to have an answer for. I think it's like a lot of medicine. How things are practiced and why they're practiced is very variable, from medication to getting PSAs, things that seem to be accepted are not often accepted, not based on the best scientific evidence. But I think it's something we all need to be aware of, and I think time will tell how well it works. But I think it's of interest.
An overview of 64-slice CT technology