The Current Scope of Artificial Intelligence in Breast US
Note: Abstract Presentation at Society of Breast Imaging Symposium, 2025.
Introduction
Breast density limits the sensitivity of mammography to detect breast lesions and is associated with an increased risk of breast cancer.1 In September 2024, the US Food and Drug Administration (FDA) mandated that all patients undergoing mammography be notified of their breast density.2 Options for supplemental screening in patients with dense breasts include breast US, breast MRI and contrast mammography. Of the 3 modalities, US is the most affordable and accessible option and does not require intravenous contrast.
However, breast US is limited by high user dependence and higher false-positive interpretations.3 As more patients receive mandatory breast density notifications, interest in supplemental screening, including breast US, will likely grow. This may drive an expanding role for artificial intelligence (AI) tools to enhance breast US performance.
This review outlines fundamental AI concepts relevant to radiologists, examines 4 breast US AI tools currently available or in development, and discusses clinical implementation challenges and future directions.
Basics of AI
AI is increasingly capable of performing tasks that traditionally required human intelligence, such as image interpretation, reasoning, and problem-solving. In radiology, AI offers the potential to enhance diagnostic accuracy and efficiency. Machine learning (ML), a core component of AI, allows systems to learn from imaging data without explicit programming. This learning can be supervised, where labeled datasets are used for model training, or unsupervised, where models identify patterns in unlabeled data. For example, a breast cancer detection and classification model may rely on input features such as lesion shape, margins, and orientation, paired with known outcomes such as “benign” or “malignant.” When new images are introduced, the model can analyze them and predict their classification.
Deep learning (DL), a more advanced subset of ML, uses multilayered neural networks to identify complex patterns in large datasets. This approach powers applications such as image recognition and natural language processing. In the context of cancer detection, DL models can analyze images to identify features indicative of malignancy, assist radiologists in detecting early-stage tumors, and improve diagnostic accuracy by reducing false positives and false negatives.4, 5
AI in Breast Imaging
The role of AI in breast imaging has advanced rapidly in recent years. ML and DL models have been applied to mammography to increase the detection of tumors. AI tools in mammography and MRI are also being investigated for their potential to evaluate image data, such as tissue patterns, and assess a patient’s risk of developing cancer.6, 7 AI tools under development have the potential to augment the work of radiologists through read-time reduction, improved detection, and more personalized cancer care delivery, especially when combined with other patient data such as genetic testing.7
Current Results of AI Algorithms in Analyzing Breast US Images
AI models applied to breast US have shown promise in improving specificity, sensitivity, efficiency, error rate, and accuracy in lesion detection and diagnosis.8 The utility of AI decision support systems (DSS) for lesion characterization and diagnostic accuracy varies depending on lesion characteristics and the specific AI tool. One FDA-cleared AI DSS demonstrated higher accuracy than radiologists in detecting irregularly shaped lesions.9 Such tools may benefit less confident readers by reducing false-positive rates.
Beyond lesion analysis, future AI algorithms may facilitate molecular subtyping of breast cancer, critical for treatment planning. Preliminary ML models have shown potential for determining molecular subtypes from breast US images.10 Other models are being developed to predict treatment response, lymph node metastasis, and survival/prognosis, offering hope for improved personalized treatment planning.10
Breast US AI Technologies
Below is a review of several AI tools developed for breast US. Owing to rapid advancements, this review cannot include all breast US AI products on the market or in development.
In addition, the authors do not have specific experience with these products, and their inclusion here should not be interpreted as endorsement. At the time of this writing, only Koios Decision Support (DS) has received FDA 510k clearance for US evaluation of lesions or nodules suspicious for breast cancer. Other vendors either have FDA clearance for more limited indications or are awaiting FDA clearance. Users should ensure they understand the approval status of any AI tool before clinical implementation.
Koios Decision Support
Koios Medical’s Decision Support (DS) is an FDA-cleared, AI/ML-based computer-aided diagnosis software device for use in breast diagnostic US. Utilizing DL algorithms, Koios DS classifies lesions using shape and orientation to assign BI-RADS categories.
In a multicenter, retrospective study, 15 readers (11 radiologists, 2 surgeons, and 2 gynecologists) evaluated 900 breast US lesions (470 benign, 430 malignant) with and without Koios DS. Mean area under curve (AUC) was significantly higher with DS alone (0.88) and US plus DS (0.87) compared with US alone (0.83, P < .0001). Interobserver agreement improved from 0.54 to 0.68 with DS, and intra-observer variability decreased from 13.6% to 10.8% ( P = .04). The effect of DS on sensitivity and specificity, however, varied with reader experience, suggesting that the tool may not offer significant accuracy improvements for experienced breast radiologists.11, 12
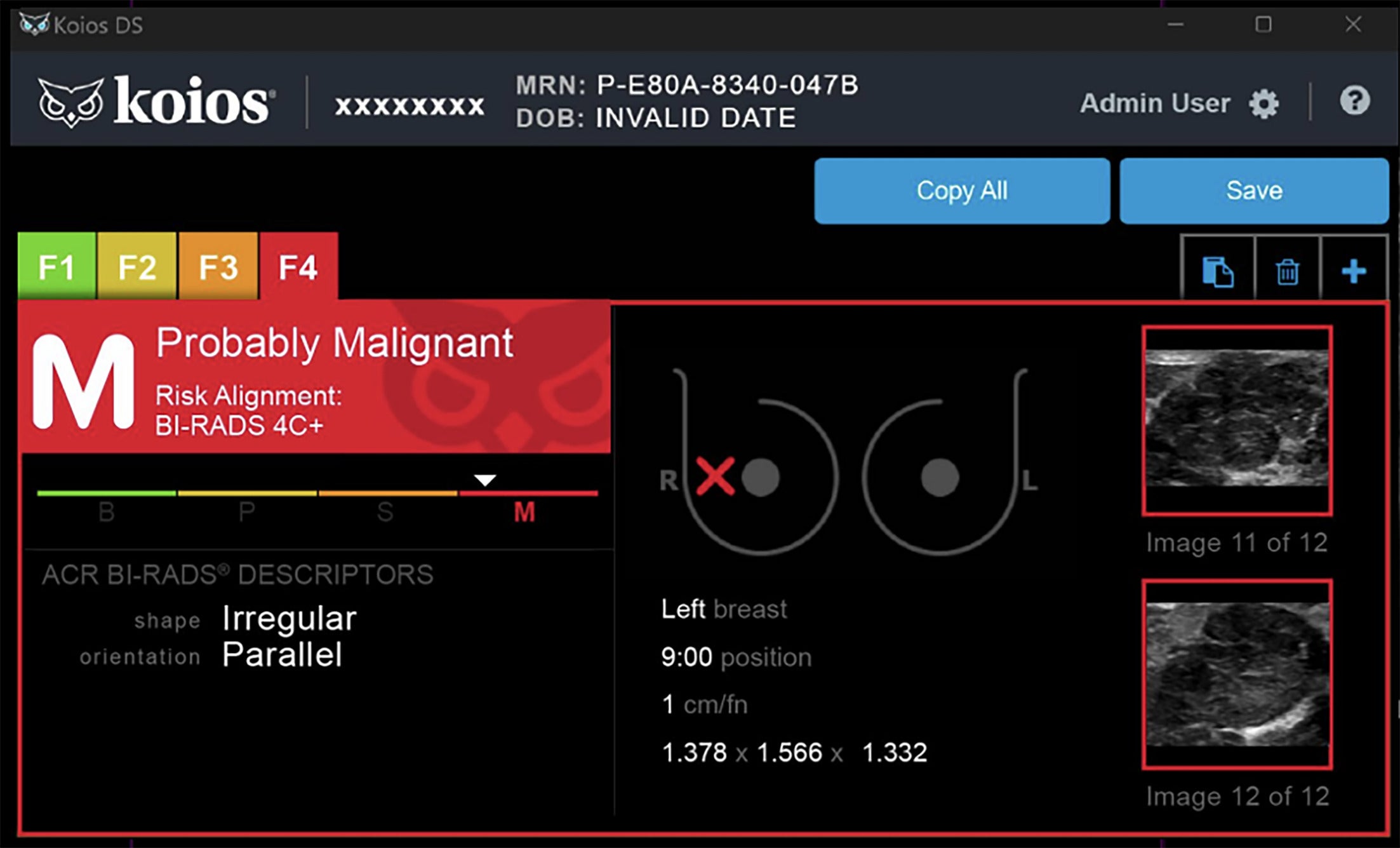
A separate retrospective analysis of the Koios AI DS tool in 75 patients (83 lesions) found 100% accuracy in identifying suspicious lesions and recommending biopsy for invasive lobular carcinoma, a diagnosis often challenging owing to subtle imaging features.13 Similarly, a single-site retrospective study of 332 patients found the Koios AI DS system identified up to 97% of triple-negative breast cancers, which can be difficult to detect due to their sometimes benign-appearing imaging features. The model’s false-negative rate in this study was comparable to that of breast radiologists. The algorithm uses parameters such as shape, direction, internal composition, and margin circumscription for lesion assessment ( Figures 1 - 3 ).14
BI-RADS 4C+. Koios artificial intelligence Decision Support interface displays the clockface location of the lesion of interest with corresponding measurements. Lesion characteristics such as shape and orientation are used as descriptors for the model to predict this lesion to be probably malignant. Images courtesy of Koios Medical, Inc.
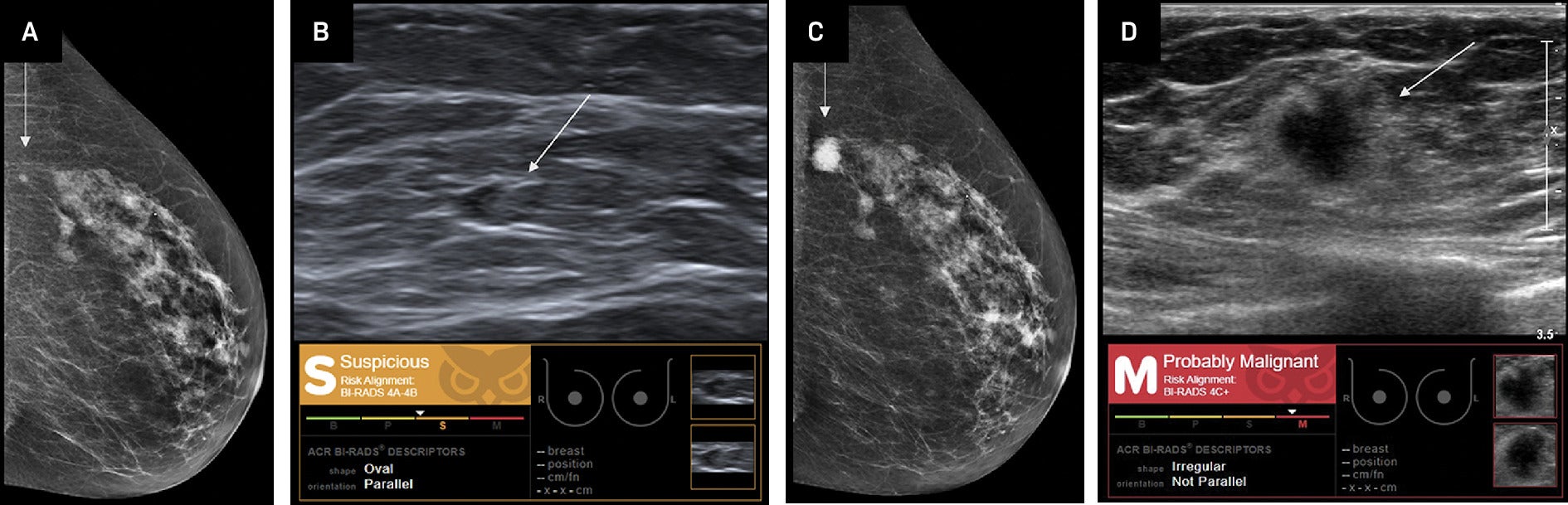
Invasive ductal carcinoma (IDC). Craniocaudal mammographic view (A) and breast US (B) demonstrate a left breast mass (arrow) that was retrospectively assessed as “suspicious,” BI-RADS category 4A-4B by Koios artificial intelligence (AI) Decision Support (DS). This mass was reported as “probably benign” by a radiologist. At the 6-month follow-up, left breast craniocaudal mammogram (C) and breast US (D) demonstrate that the mass (arrow) has increased in size. The mass is assessed as “probably malignant,” BI-RADS category 4C+ by Koios AI DS owing to its irregular shape and nonparallel orientation. Biopsy demonstrated triple-negative IDC. Images from Coffey et al.14 Images courtesy of Koios Medical, Inc.
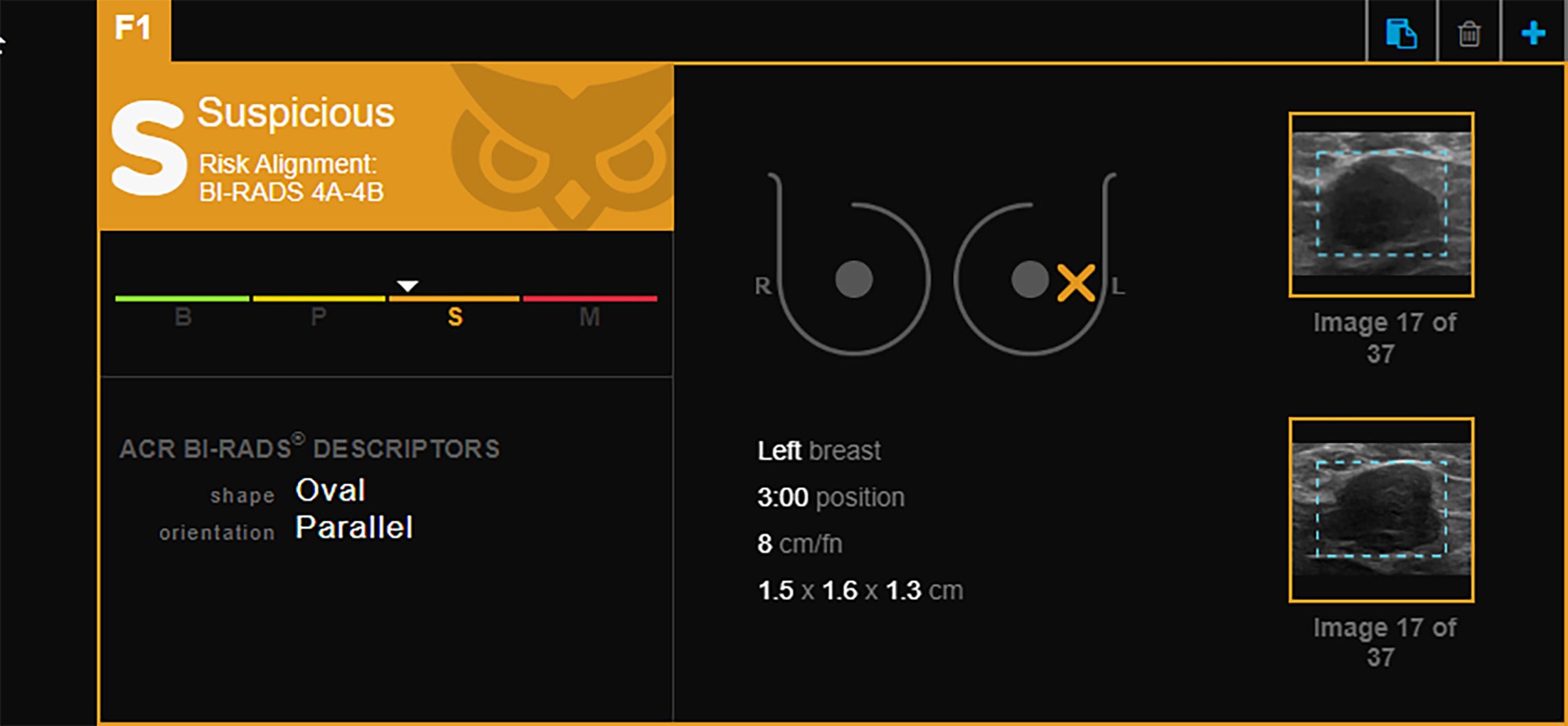
Invasive ductal carcinoma (IDC). Retrospective Koios artificial intelligence Decision Support categoric assessment of lesions previously identified as IDC via breast US-guided biopsy. White triangle marker position indicates the level of confidence in the assignment in the “suspicious” BI-RADS category 4A-4B. Images courtesy of Koios Medical, Inc. and Victoria Mango, MD.
In another assessment of Koios AI DS, a multicenter retrospective study of 715 breast masses found that none of the BI-RADS-2 assignments made with AI assistance were later identified as malignant. This approach contributed to an 11% reduction in benign biopsies within the study population.15
See-Mode
See-Mode has developed breast US analysis software, currently under FDA review, to analyze lesion shape, orientation, margins, echogenicity, and posterior features to generate a BI-RADS assessment category. To account for variations in image acquisition and patient characteristics, model training incorporates site-specific and regional data.
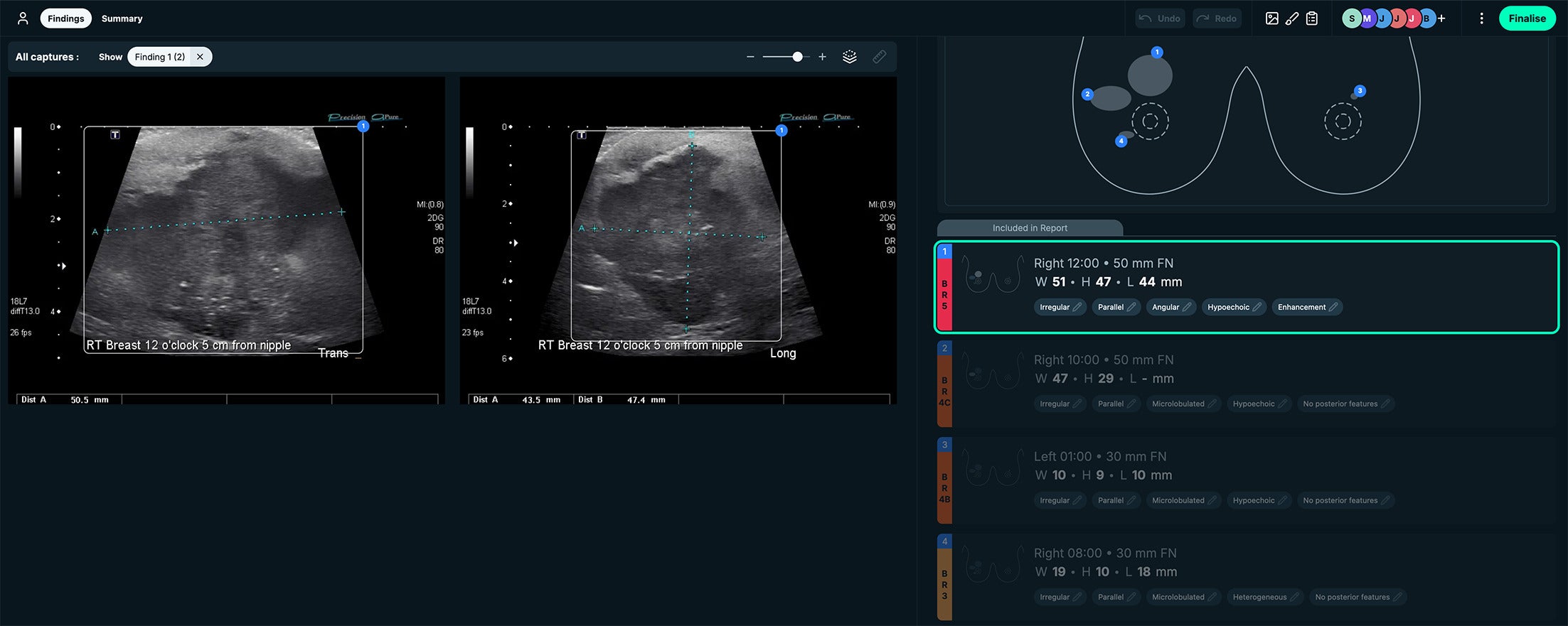
The See-Mode software highlights suspected lesions with visual markers such as colored overlays and bounding boxes, along with measurements. A standardized clock face design is also included in the interface for lesion localization ( Figures 4 - 6 ).
BI-RADS 5. See-Mode’s interface displays the clockface location of the lesion of interest and displays measurements side by side with the US images. This case was a BI-RADS 5 classification, owing to irregular shape, angular margins, and hypoechoic echo pattern. Images courtesy of See-Mode.
BI-RADS 3. See-Mode’s interface displays lesion measurements side by side with the US images. This case was a BI-RADS 3 classification due to irregular shape, angular margins, and complex cystic and solid appearance. Images courtesy of See-Mode.
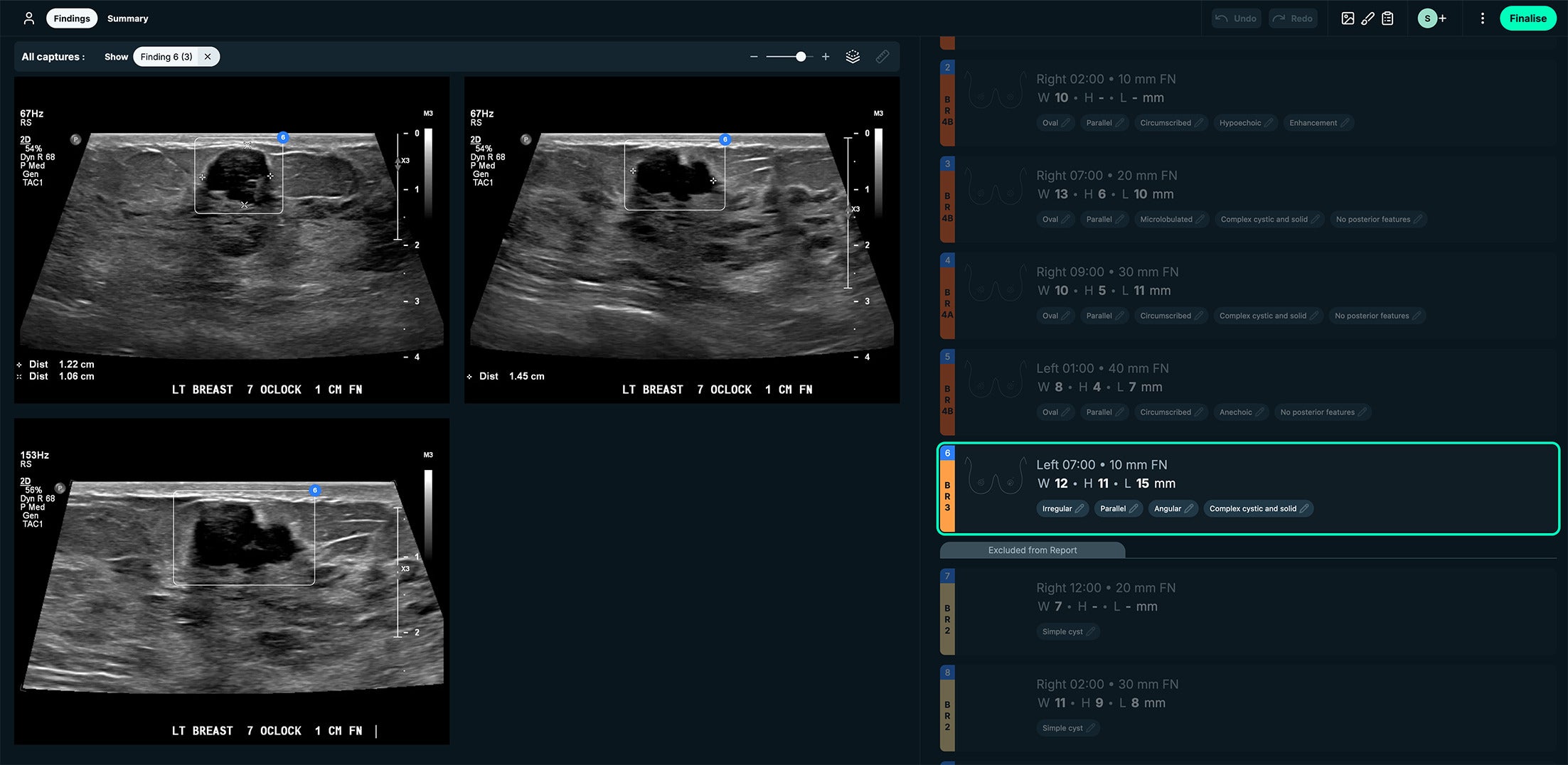
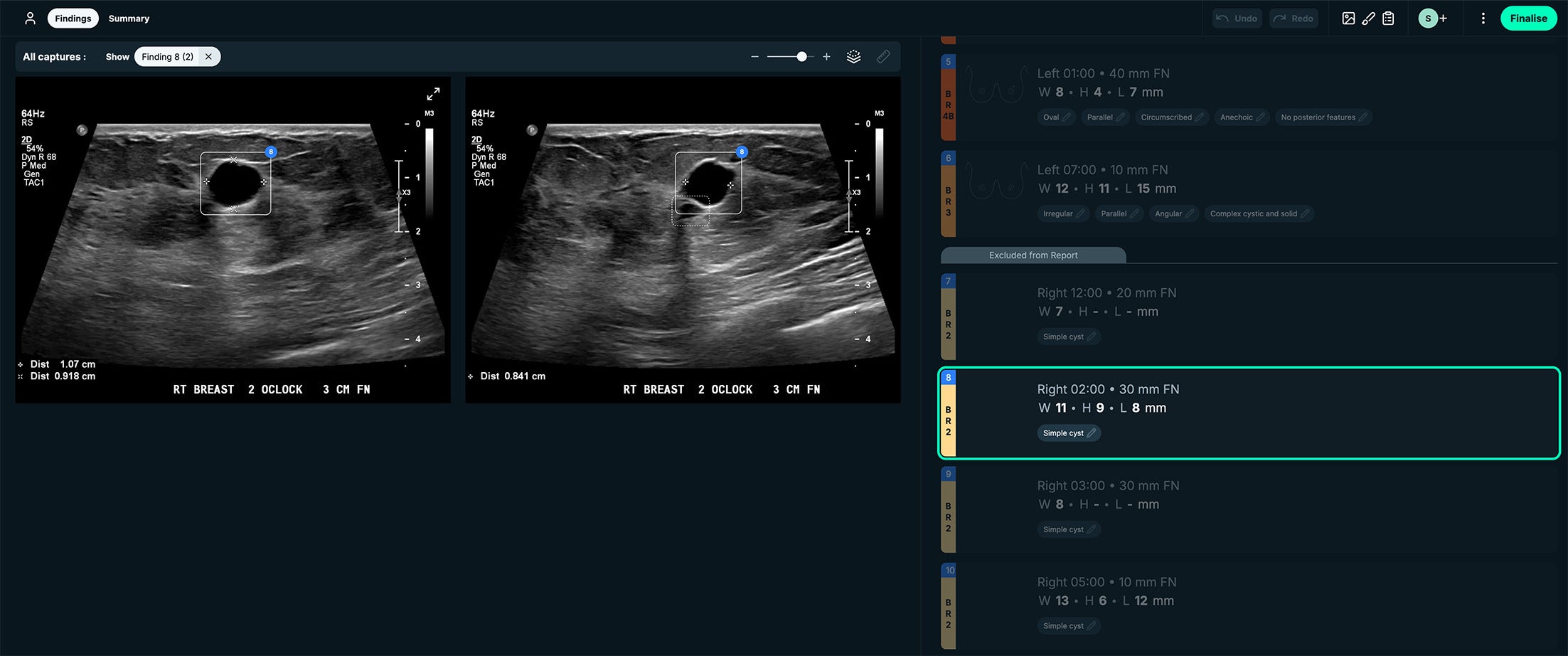
BI-RADS 2. See-Mode’s interface displays lesion measurements side by side with the US images. This case had a simple cyst appearance. Images courtesy of See-Mode.
S-Detect
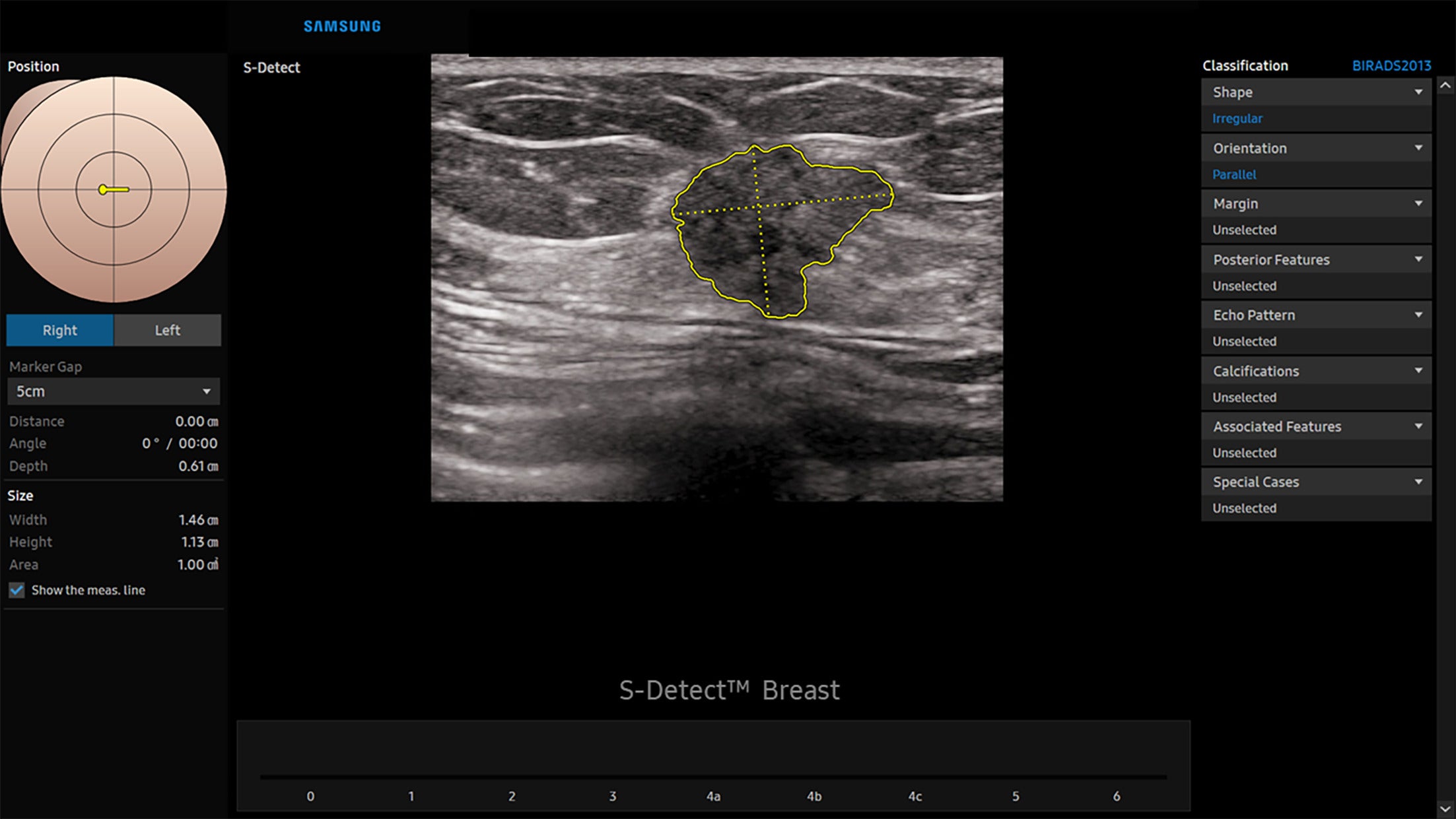
Samsung’s S-Detect for Breast is designed to assist radiologists during US imaging. Currently available for use on Samsung US systems, the software analyzes lesion shape, orientation, margins, echogenicity, posterior features, and calcifications to generate a BI-RADS assessment category ( Figure 7 ).
Irregular mass. S-Detect artificial intelligence Decision Support creates a lesion map with position and measurements on the left side of the figure, US image in the center, and selected classification features on the right. This mass was classified as irregular shape and parallel orientation. Images courtesy of Samsung Electronics.
One study compared S-Detect’s performance to radiologists with varying experience levels. Analyzing 206 breast masses (118 benign, 88 malignant), S-Detect demonstrated 91.1% sensitivity, 80.1% specificity, and 85.4% overall accuracy. Radiologists using S-Detect showed improved specificity (from 76.2% to 84.4%) and slightly increased accuracy (from 85.4% to 88.3%), suggesting that S-Detect may enhance diagnostic assessments.16
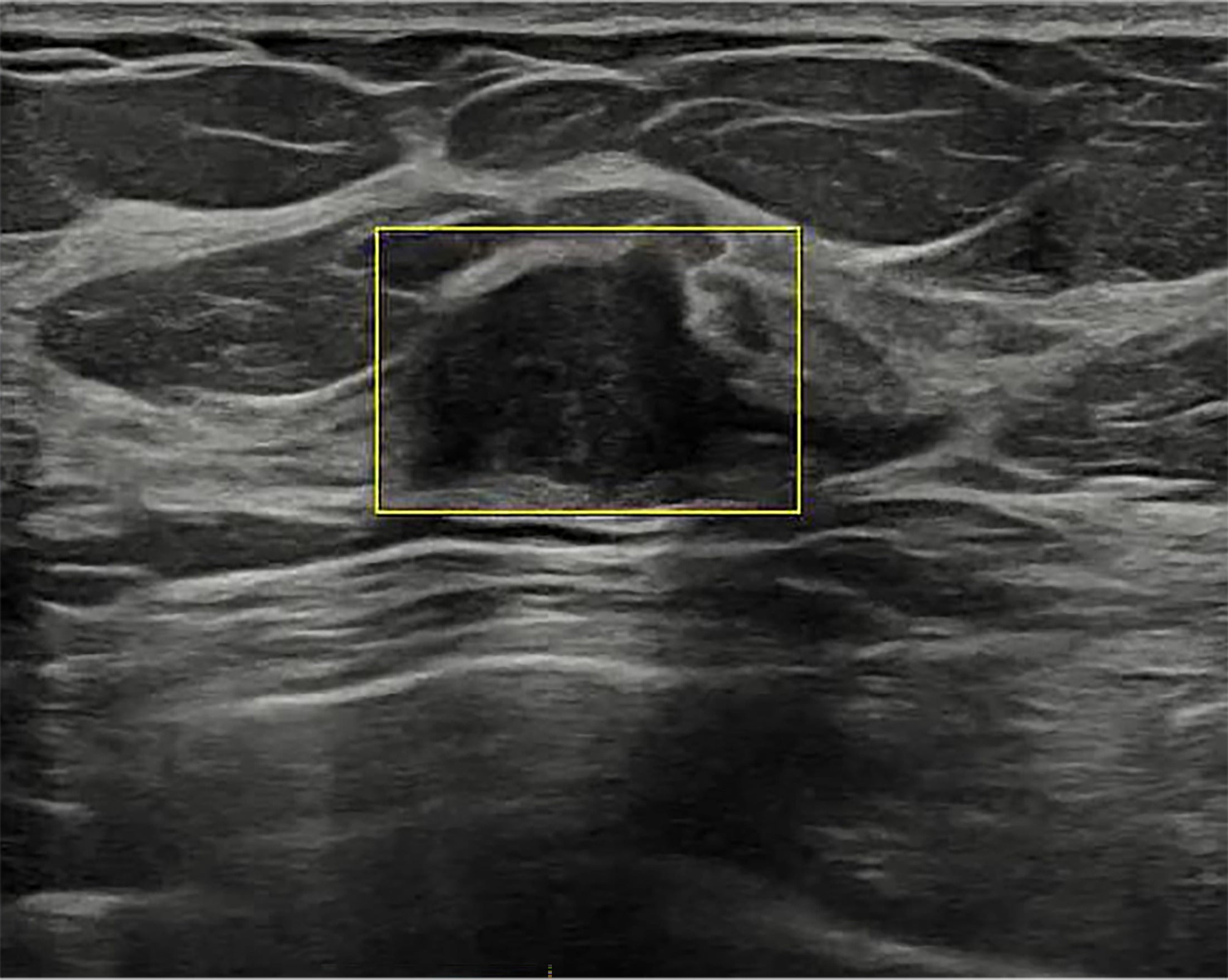
S-Detect’s “Live Breast Assist” feature ( Figure 8 ), currently under FDA review, uses DL to detect areas of interest in real time during scanning. This feature supplements the sonologist’s lesion detection by automatically highlighting potential abnormalities.
Artificial intelligence (AI) lesion detection. S-Detect AI Decision Support has a US Food and Drug Administration-pending feature that detects potentially abnormal findings and displays a yellow box to highlight the finding to the sonologist during live US scanning of the breast. Images courtesy of Samsung Electronics.
BU-CAD
Breast Ultrasound Computer-Aided Diagnosis (BU-CAD), developed by TaiHao Medical Inc, is designed to analyze 2D and 3D images. The system evaluates lesion shape, orientation, margins, echogenicity, posterior features, and calcifications ( Figures 9 - 11 ) and generates automated BI-RADS categorizations. A study evaluating BU-CAD using a dataset of 14,624 images from 7516 patients across multiple centers reported a 13.6% increase in specificity without compromising sensitivity, along with lower inter-reader variability.17
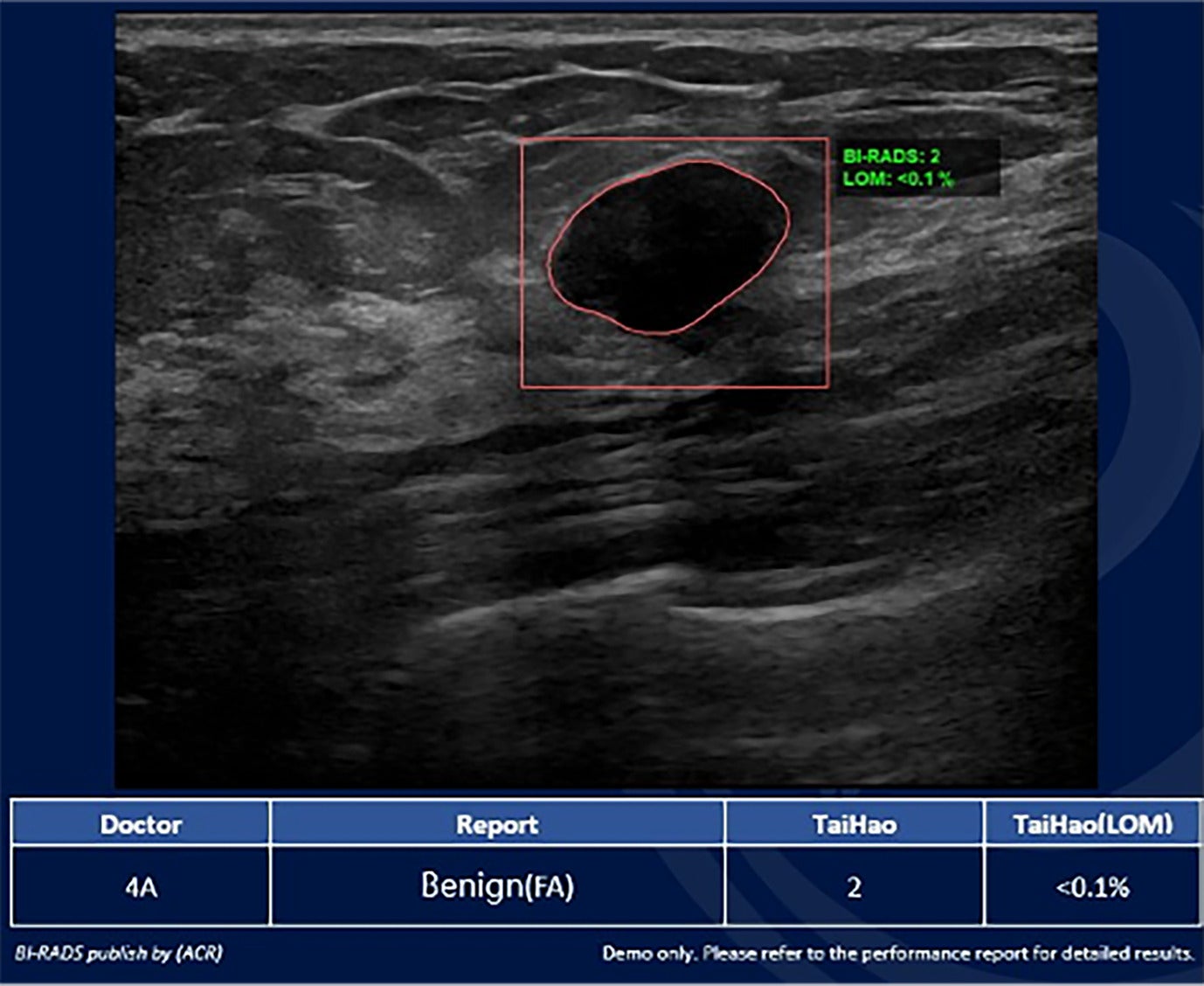
BI-RADS 2. Breast Ultrasound Computer-Aided Diagnosis interface showing a navigation bar on the left and an US image with the outlined lesion in the center. The right side displays a map of the probe on the breast (top right) with clock face coordinates below. This mass was categorized as BI-RADS 2, likelihood of malignancy < 0.1%. Image courtesy of TaiHao Medical Inc.
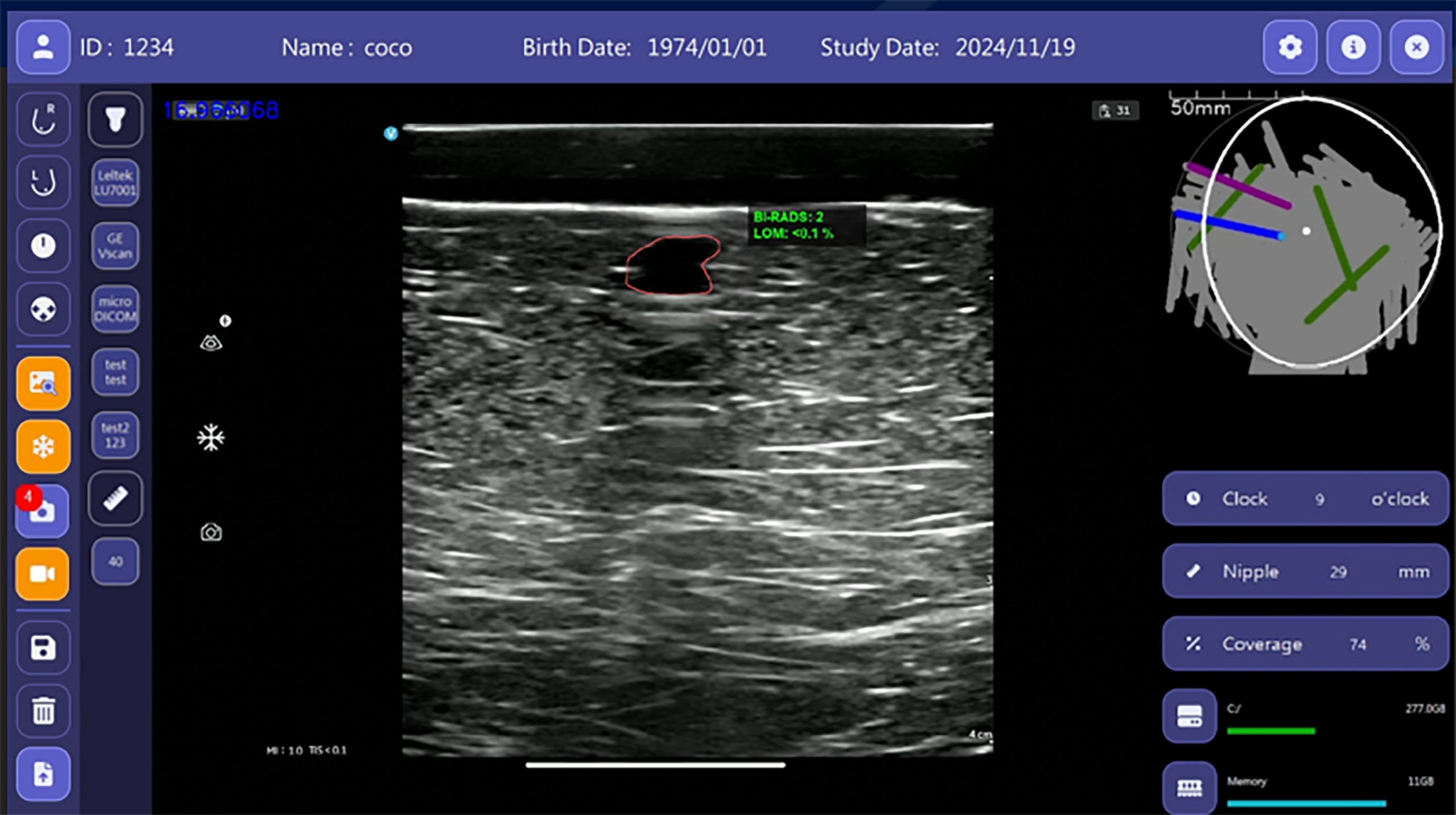
BI-RADS 2. Breast Ultrasound Computer-Aided Diagnosis interface with the US image of the outlined lesion with accompanying measurements in the center. This mass was categorized as BI-RADS 2 based on its oval shape, parallel orientation, and circumscribed margins. Image courtesy of TaiHao Medical Inc.
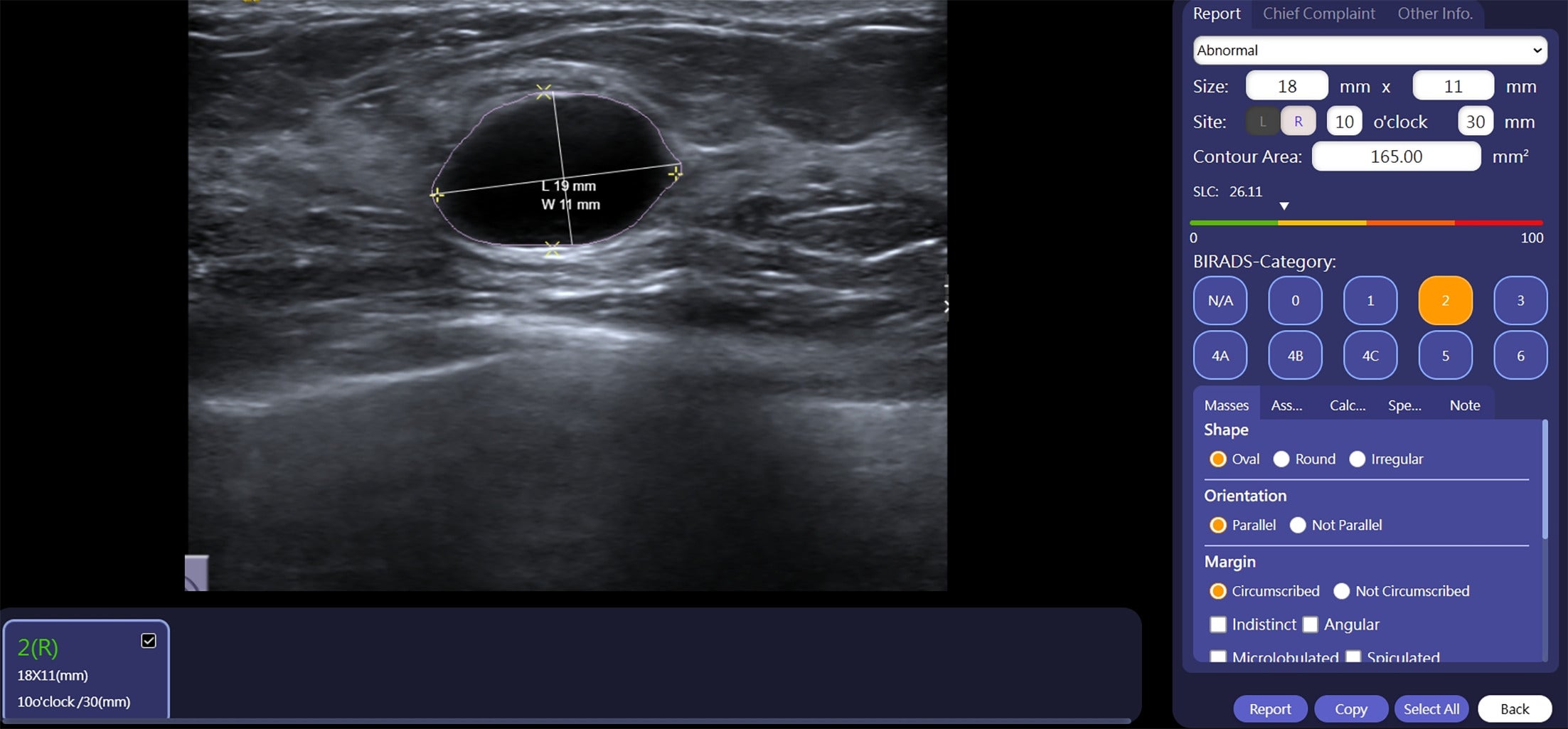
Artificial intelligence/radiologist discrepancy. Breast Ultrasound Computer-Aided Diagnosis (BU-CAD) interface with the US image of the outlined lesion. This mass was categorized as BI-RADS 2, <0.1% likelihood of malignancy (LOM). At the bottom, the radiologist’s assignment of BI-RADS 4A and TaiHao’s BU-CAD assignment of BI-RADS 2 are displayed. This mass demonstrated benign pathology via fine needle aspiration. Image courtesy of TaiHao Medical Inc.
Summary of AI Tools for Breast US
Each of these AI tools offers distinct features to aid in breast lesion detection and diagnosis ( Table 1 ). They vary by the specific morphological features they use to characterize masses, but most characterize findings already identified by the sonologist. While each tool has its strengths, their differences may indicate different suitability for varied clinical and workflow needs.
Chart Summarizing the Capabilities of 4 Currently Available Breast US Artificial Intelligence Tools: Koios, See-Mode, S-Detect, and BU-CAD Systems
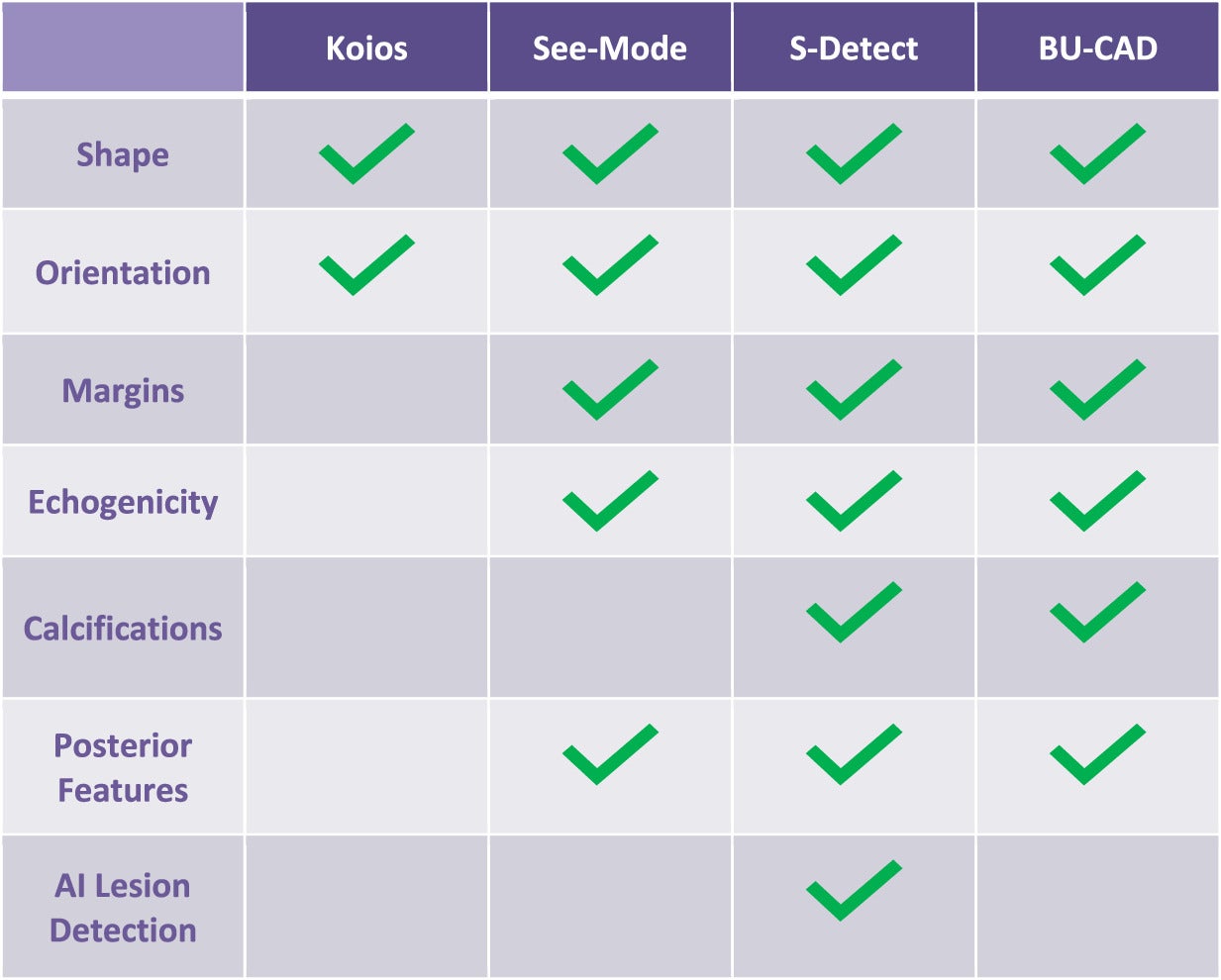 |
Limitations, Challenges, and Next Steps
The future of breast US in clinical practice is likely to include AI to enhance lesion analysis and aid cancer detection. There are, however, challenges to overcome before AI earns widespread clinical implementation.
First, AI tools will require appropriate governance and monitoring. Many studies to date are limited to single institutions and do not reflect large or diverse study samples, potentially limiting local applicability in clinical practices.6 Pre-validation in the local environment is needed to ensure local model performance is on par with expectations from prior studies. Training bias exists, and local performance will be affected by training datasets that differ from local datasets.
Following clinical deployment, monitoring of algorithm performance is important; assessments must be performed to detect algorithm drift. Algorithm drift can degrade a radiology model’s performance after training due to evolving factors such as patient demographics, imaging equipment/protocols, and the emergence of new diseases or variants.
Second, research is needed to evaluate the impact of breast US AI across clinical environments and practitioners of varying levels of US expertise. AI-based analysis tools may assist technologists and radiologists with less experience. However, reliance on them could increase false-positive findings.
Importantly, the success of AI tools relies on practitioner and patient acceptance. AI algorithms are so-called “black box” solutions that lack transparency in their decision-making processes, potentially hindering their uptake by radiologists.18 Patient sentiment should also be considered. In one study, half of the women above screening age viewed AI analysis of their breast images favorably, with the other half viewing it with negative and/or neutral sentiments.19 Education of all stakeholders regarding the benefits and limitations of AI will be an important factor in the successful integration of these technologies into breast imaging.
Conclusion
The FDA’s breast density notification mandate is driving increased demand for supplemental breast cancer screening, including breast US, and creating a critical need for AI tools to support successful and efficient implementation.
For radiologists, effectively integrating AI into practice requires a foundational understanding of the technology’s principles. This consists of critically evaluating published performance metrics (eg, sensitivity, specificity, area under the curve) in the context of one’s own practice, becoming aware of potential biases in training data (and the resulting limitations of AI models), and understanding the potential for algorithm drift.
While they vary in their features and functionalities, the tools discussed in this review share the goal of enhancing lesion detection and characterization. By actively engaging with these advancements, radiologists can potentially optimize diagnostic accuracy and workflow efficiency in breast US. Further research, including prospective studies and rigorous clinical validation, is crucial to fully realize the potential of AI in breast US.
References
Citation
Tafreshi R, Panjwani B, Goldberg-Stein SA, Barish M, Park J, Vincoff NS.The Current Scope of Artificial Intelligence in Breast US. Appl Radiol. 2025; (2):1 - 9.
doi:10.37549/AR-D-25-0074
April 16, 2025