Nuclear medicine of the painful joint replacement
Images







Dr. Palestro is Professor of Nuclear Medicine and Radiology, Albert Einstein College of Medicine, Bronx, and Chief of the Division of Nuclear Medicine, Long Island Jewish Medical Center, New Hyde Park, NY. Dr. Love is a Research Scientist, Division of Nuclear Medicine and Dr. Marwin is an Associate Chairman, Department of Orthopedic Surgery, Long Island Jewish Medical Center, New Hyde Park, NY.
Although most of the nearly 500,000 joint replacements performed annually in the United States are successful, complications do occur. While many such complications are easily diagnosed, differentiating aseptic loosening from infection can be a daunting task since these 2 entities are remarkably similar, clinically and histopathologically. This article reviews the clinical aspects of prosthetic joint failure, the appearances of aseptic loosening and infection on various radionuclide imaging studies, and the advantages and disadvantages of these procedures.
The era of modern joint replacement surgery is <50 years old. The modern hip prosthesis, the prototype of which was developed in the late 1950s, is a modular appliance that permits the surgeon to modify the components to suit an individual patient's needs. A total hip arthroplasty consists of femoral and acetabular components. An arthroplasty in which the femoral component articulates with the native acetabulum is a hemiarthroplasty.
The predecessor of the modern knee replacement, developed in the mid-1970s, was a fixed-bearing implant consisting of a 1-piece metallic femoral component, a polyethylene patellar component, and a polyethylene tibial tray with a central peg. Its modern, mobile-bearing descendants offer superior joint mobility with less polyethylene breakdown. 1
In addition to polymethylmethacrylate (PMMA), or surgical cement, there are several ways by which these devices, composed of metal (cobaltchromium and titanium), and an ultra-high molecular-weight (UHMW) polyethylene plastic, can be secured to native bone. Cementless, porous-coated prostheses depend on bony ingrowth into a porous coating applied to the surface of the device. Some prostheses are coated with hydroxyapatite compound, which stimulates new bone formation and serves as an attachment for newly formed osseous tissue around the hardware. Acetabular components can be forced (press-fit) into the acetabulum or secured with orthopedic screws. 1,2
Prosthetic failure
By 10 years after implantation, rough- ly half of all prostheses exhibit radio-graphic evidence of loosening and up to 30% require revision. 3,4 In many cases, loosening results from an inflammatory or immune reaction. 5 A synovial-like pseudomembranous structure develops. The cellular composition of the pseudo-membrane is varied: histiocytes are seen most often (95% of specimens), followed by giant cells (80%), and lymphocytes and plasma cells (25%). Neutrophils are present in <10% of the cases. 6 Particulate debris produced by component fragmentation attracts and activates local tissue phagocytes. This debris is impervious to enzymatic destruction, leading to re-peated, unsuccessful, attempts at phagocytosis, which, in turn, stimulate secre- tion of proinflammatory cytokines and proteolytic enzymes that damage bone and cartilage and activate immune cells. This process leads to osteolysis with loss of supporting osseous tissues and prosthetic loosening. 5-7
Infection affects <2% of primary and <5% of revision arthroplasties. Bacteria readily bind to the materials used in joint arthroplasties, and, once attached to the implant, some bacteria produce a surface glycocalyx that protects them from the host inflammatory response. About one third of prosthetic joint infections develop within 3 months, another third within 1 year, and the remainder >1 year after surgery. The inflammatory reaction accompanying the infected prosthesis is similar to that of aseptic loosening, with one important difference: neutrophils, usually absent in aseptic loosening, which are invariably present in large numbers in infection. 8-11
Treatment of prosthetic infection often requires multiple admissions. An excisional arthroplasty is performed, followed by antimicrobial therapy, and eventually, revision arthroplasty. Aseptic loosening, in contrast, is usually managed with a single-stage exchange arthroplasty that requires only 1 hospital admission and 1 surgical intervention. 1
Because their treatments are so different, the importance of distinguishing infection from aseptic prosthetic loosening cannot be overstated. This distinction, unfortunately, can be difficult. Clinical signs of infection are often absent and laboratory tests are unreliable. The results of joint aspiration have been disappointing, with large numbers of false-positive and false-negative results reported. Radio-graphs are neither sensitive nor specific, and hardware-induced artifacts limit the utility of cross-sectional imaging modalities. Radionuclide or functional imaging studies are not hindered by metallic hardware and are very useful for evaluating the painful joint replacement. 1,12-15
Radionuclide imaging
Bone scintigraphy
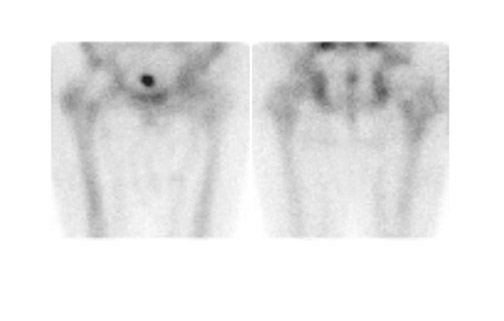
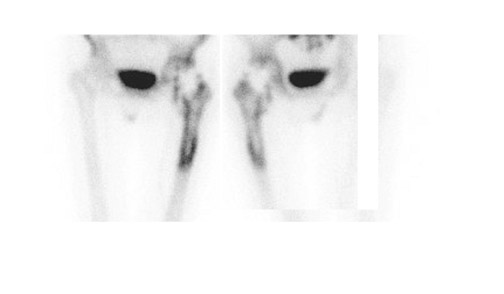
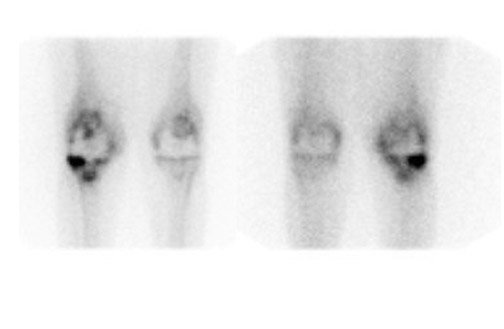
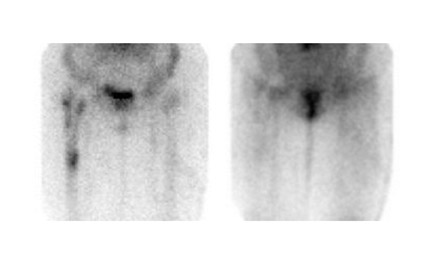
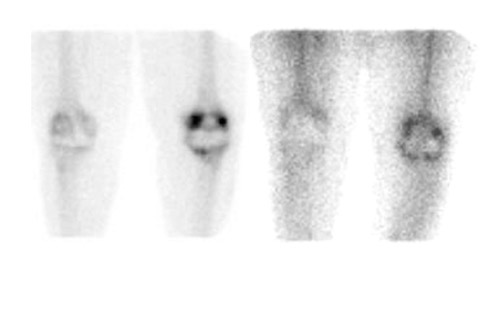
Bone scintigraphy is widely available, easily performed, and extremely sensitive. A normal study-ie, one in which periprosthetic uptake is indistinguishable from surrounding nonarticular bone-rules against a prosthetic abnormality (Figure 1). The significance of increased periprosthetic uptake, however, is less certain. Several early investigations found that the test could identify the failed joint replacement, but could not determine the cause of failure. 16-18 Merely confirming prosthetic failure is not sufficient; the cause of the failure must be determined if appropriate treatment is to be instituted. This is especially true today, when many patients are referred to nuclear medicine specifically to differentiate aseptic loosening from infection. Some investigators have suggested that aseptic loosening of hip prostheses can be distinguished from infection by analyzing periprosthetic up-take patterns. Williamson et al 19 reported that focal periprosthetic uptake was as-sociated with loosening, while diffuse uptake around both the femoral and ace-tabular components was associated with infection. Williams et al, 20 however, found that diffusely increased activity was associated with both aseptic loosening and infection (Figures 2 and 3). Mountford et al 21 found that this pattern was reasonably specific, but not sensitive for infection. Aliabadi et al 22 reported that bone scintigraphy was moderately sensitive and very specific for diagnosing loosening with or without infection, but could not distinguish the loosened uninfected from the loosened infected prosthesis. Lieberman et al 23 reported that bone scintigraphy was sensitive and specific for identifying loosened hip replacements but excluded infected devices from analysis.
The age of the prosthesis, together with the introduction of new types of prostheses, further complicates interpretation of bone scintigraphy. During the first year after prosthetic hip implantation, periprosthetic uptake patterns are variable and only a normal bone scan is reliable. In cemented hip replacements >1 year old, persistent periprosthetic uptake is present in up to 10% of asymptomatic devices. 24 Persistent uptake beyond 1 year is even more frequent in cementless or porous-coated hip replacements. 25-27 Few, if any data are available about the evolution of periprosthetic uptake patterns in other types of hip prostheses.
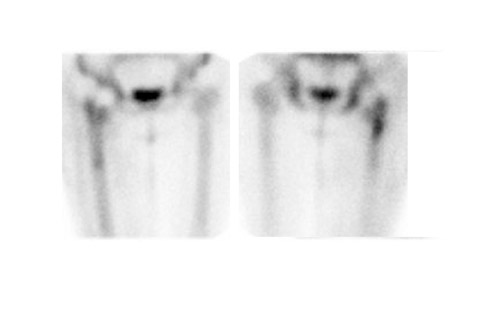
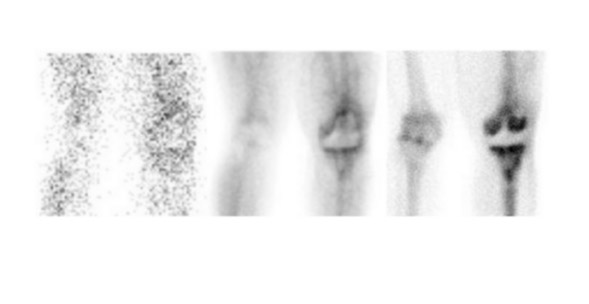
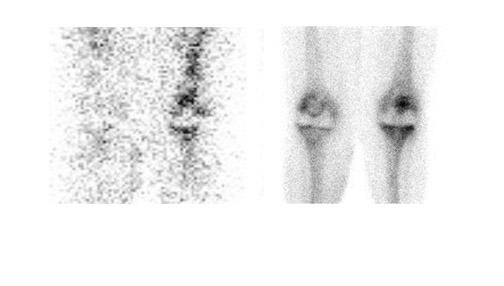
Evaluating knee replacements is even more problematic, as increased periprosthetic activity can persist for some time after implantation. 28-30 Hofman et al 30 studied asymptomatic knee replacements with serial bone scans over 2 years. They concluded that, although periprosthetic activity generally decreased over time, there was considerable patient-to-patient variation, and sequential scans are needed to determine the significance of increased periprosthetic uptake (Figure 4).
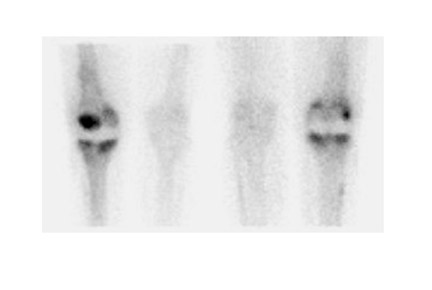
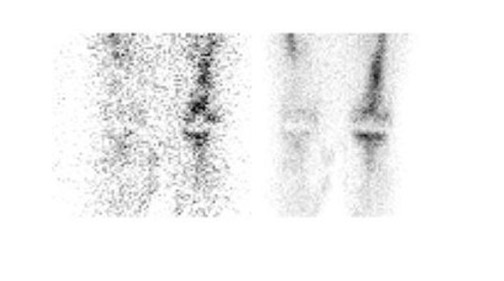
Further complicating matters is the fact that most joint replacement infections occur within 1 year after implantation when periprosthetic uptake is so variable that only a normal bone scan contributes useful information, regardless of the type or location of the prosthesis. Three-phase bone scintigraphy does not improve the accuracy of bone scintigraphy, which is approximately 50% to 70% (Figure 5). 29,31,32
In summary, bone scintigraphy is sensitive, with a high negative predictive value, and is useful as a screening test.
Bone/gallium imaging
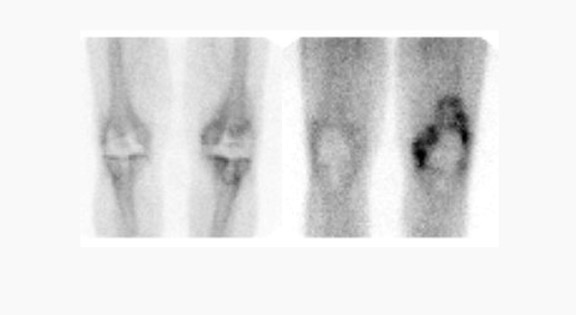
Gallium-67 citrate imaging is often performed together with bone scintigraphy to diagnose musculoskeletal infection. Uptake mechanisms of the diphos- phonates and gallium are different, and each study provides complementary information about different aspects of a disease process. The combined study is positive for infection when the distribution of the 2 radiotracers is spatially incongruent, or when their distribution is spatially congruent and the intensity of gallium uptake exceeds that of the diphosphonate (Figure 6). The combined study is equivocal for infection when the distribution of the 2 tracers is spatially congruent and the relative intensity of uptake of each tracer is similar (Figure 7). The combined study is negative for infection when gallium images are normal, regardless of the bone scan findings, or when the spatial distribution of the 2 tracers is congruent and the relative intensity of gallium uptake is less than that of the diphosphonate (Figure 8). 33
Although Tehranzadeh et al 34 reported 95% accuracy for the combined study, other investigators have reported less satisfactory results. Merkel et al 35 found that the sensitivity, specificity, and accuracy of the technique for diagnosing joint replacement infection in a canine model were 61%, 71%, and 67%, respectively. In 130 patients with painful orthopedic prostheses, these investigators reported that bone/gallium imaging was 66% sensitive, 81% specific, and 77% accurate for diagnosing infection. 36 Gomez-Luzuriaga et al 37 reported a sensitivity, specificity, and accuracy of 70%, 90%, and 80%, respectively, for bone/gallium imaging. Kraemer et al 38 reported 38% sensitivity and 100% specificity for bone/gallium imaging for diagnosing prosthetic hip infection. With an accuracy that ranges from roughly 60% to roughly 80%, bone/gallium scintigraphy offers only a modest improvement over bone scintigraphy alone.
Labeled leukocyte scintigraphy
The accuracy of labeled leukocyte (white blood cell [WBC]) imaging for diagnosing prosthetic joint infection has been the subject of controversy. Some investigators have found the study to be reasonably sensitive and specific. 31,39-41 Other investigators have reported that the test is specific, but not sensitive, attributing poor sensitivity to the chronicity of the process. 21,42 Still other investigators have found that labeled leukocyte imaging is sensitive but not specific, attributing poor specificity to nonspecific in-flammation. 43,44
The accuracy of WBC imaging is related to the presence of neutrophils. The paucity of neutrophils in the aseptically loosened prosthesis, together with their invariable presence in infected hardware, rules against chronicity and nonspecific inflammation as explanations for the poor results reported by some investigators. The explanation is, in fact, related to image interpretation. WBC images are interpreted as positive for infection when the intensity of uptake in the region of interest exceeds that in some reference point, or when activity outside the normal distribution of the tracer is observed (Figure 9). The intensity of labeled leukocyte uptake in a focus of infection-as well as the normal distribution of labeled leukocytes-is variable. 1 In one investigation, the sensitivity and specificity of WBC imaging for diagnosing infected hip replacements varied with the interpretive criteria used. When any periprosthetic activity was considered positive for infection, the test was 100% sensitive and 23% specific. When only activity more intense than the contralateral side was considered positive for infection, specificity rose to 61% and sensitivity fell to 65%. 45 These investigators observed similar changes in spe-cificity when these same criteria were applied to knee replacements. 29
Labeled leukocytes accumulate in the reticuloendothelial cells, or fixed macro-phages of the bone marrow, the normal distribution of which closely parallels that of the hematopoietically active marrow, which is limited to the axial skeleton and proximal humeri and femurs in normal adults. White blood cell activity outside these regions is often interpreted as indicating infection. 46 There is, however, considerable individual-to-individual var-iation in the distribution of hematopoietically active (and, hence, the reticuloendothelial) marrow. Generalized marrow expansion is a response to a systemic process, such as anemias, tumors, and other myelophthisic states. Localized marrow expansion is a response to local stimuli, such as fracture, inflammation, orthopedic hardware, a Charcot joint, and even calvarial hyperostosis. 46-48 Generalized and localized marrow expansion alters the "normal" distribution of marrow making it difficult to separate WBC accumulation in atypically located, but otherwise normal, marrow from uptake in infection.
Some investigators have found that interpreting WBC images together with radionuclide bone images improves the accuracy of the study. This improvement was the result of improved specificity, which came at the expense of sensitivity. 43,44 Other investigators have found the technique less useful. 25,29 In one investigation of total knee replacements, the sensitivity and specificity of WBC/bone imaging were no better than those of WBC imaging alone 29 (Figure 10). Oswald et al 25 reported that WBC bone images were incongruent in 15% of asymptomatic porous-coated hip arthroplasties. One problem with WBC/ bone imaging is that while diphosphonates accumulate in bone, labeled leukocytes accumulate in marrow. Conditions affecting marrow may or may not affect bone and vice versa. Even when an entity affects both bone and marrow, the effects may be dramatically different on each. 49
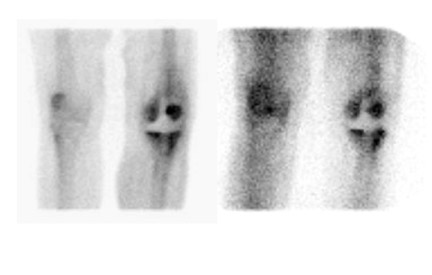
Several investigators have used WBC imaging together with sulfur colloid (marrow) imaging for diagnosing the infected joint replace- 29,45,50-52 The distribution of marrow activity on WBC and marrow images is similar in normal individuals as well as in those with underlying marrow abnormalities, ie, the images are spatially congruent. The exception to this is osteomyelitis, which exerts opposite effects on these 2 tracers. While stimulating the uptake of leukocytes, osteomyelitis suppresses uptake of sulfur colloid. In osteomyelitis, therefore, the distribution of activity on WBC and marrow images is different. The WBC/marrow study is positive for infection when there is activity on the WBC image without corresponding activity on the marrow image-ie, the images are spatially incongruent. When any other pattern is present, the study is negative for infection (Figures 11 and 12). 46 The accuracy of WBC/marrow imaging ranges from 88% to 98%. 29,45,50-52
Meticulous technique is critical to the success of WBC/marrow scintigraphy. When the study is performed with indium-111-labeled leukocytes, marrow imaging can be performed either before or after WBC imaging, or as a simultaneous dual isotope study. The use of technetium (Tc)-labeled leukocytes neces- sitates modifications. Since both parts of the study employ the same radionuclide, 99m Tc, at least 48 hours should elapse between the 2 tests. Sulfur colloid should be prepared just before use. Using sulfur colloid that is more than approximately 1 to 2 hours old may result in images of inferior quality, including increased background and urinary bladder activity, which is especially troublesome when studying the hip. 1
Other agents
In-vitro WBC labeling is labor-intensive, is not always available, and requires direct contact with blood products. The need for marrow imaging adds to the complexity and cost, and is inconvenient for patients. Thus, investigators continue to search for suitable alternatives. Although in-vivo WBC labeling techniques have been explored, none of them are currently available in the United States.
Although initial reports suggested that fluorodeoxyglucose positron emission tomography (FDG-PET) could accurately identify the infected joint prosthesis, recent studies are less encouraging. 52-56 Fluorodeoxyglucose PET does not ap-pear to be capable of distinguishing the inflamed aseptically loosened prosthesis from the infected prosthesis (Figure 13). 52 This is not surprising when one considers that FDG uptake depends on tissue metabolism. Inflammation and infection are hypermetabolic states, and both will manifest as areas of increased activity on FDG PET images.
A novel approach to infection imaging is the use of radiolabeled antibiotics. The most extensively investigated tracer in this group is the radiolabeled fluoroquinolone, 99m Tc-ciprofloxacin. Although early investigations indicated that 99m Tc ciprofloxacin is moderately sensitive and very specific for infection, more recent data suggest that, in orthopedic infections, this agent may be more sensitive than specific. 57-59 Sarda et al 59 re-ported that, although the agent was sen- sitive, it could not discriminate between infected and aseptic osteoarticular disease. This study did not specifically address prosthetic joints, but the findings are of concern and point to the need for focused investigations in this area (Figure 14).
Conclusion
The primary role of nuclear medicine for a painful joint replacement-differentiating aseptic loosening from infection of a prosthetic joint- remains a daunting task. The relationship between aseptic loosening and inflammation limits the role of nonspecific indicators of inflammation, such as bone scintigraphy and FDG-PET, to that of a screening test. For the moment at least, WBC/marrow imaging, despite its disadvantages, remains the procedure of choice for diagnosing infected joint replacement.
Related Articles
Citation
Nuclear medicine of the painful joint replacement. Appl Radiol.
November 14, 2006