MRI determination of myocardial viability
Images

Dr. Grand is an Instructor, and Dr. Bluemke is an Associate Professor of Radiology and Medicine and the Clinical Director of MRI, Russell H. Morgan Department of Radiology and Radiological Sciences, The Johns Hopkins University School of Medicine, Baltimore, MD.
Disclosure: Gadolinium-enhanced MRI of the heart is off-label use of a contrast agent.
In the past decade, great strides have been made in magnetic resonance imaging (MRI) of the heart and cardiovascular system. Faster pulse sequences as well as extensive clinical and laboratory research have rendered MRI an increasingly important tool in the cardiac imaging arsenal. Cardiac MRI can provide the clinician with valuable structural, anatomic, and functional information that directly impacts subsequent patient management.
As a single examination, MRI is exceptionally versatile; however, it may have found its most important cardiac application in the determination of myocardial viability. The goal of diagnostic tests to determine myocardial viability is to distinguish myocardium that has the ability to contract versus myocardium that is replaced by fibrosis or scar. MRI is rapidly becoming the clinical gold standard for this assessment.
Background
Approximately 3 decades ago, it was noted that regions of abnormal myocardial function could recover contractility after revascularization. Two subtypes of abnormally functioning myocardium that retain the potential for recovery have subsequently been described. Segments that lose function as a result of an acute ischemic insult despite the restoration of normal perfusion are known as stunned myocardium . These segments have a mismatch between flow and function and are likely to recover function spontaneously over time. Hibernating myocardium is the term used to refer to segments rendered dysfunctional secondary to chronic ischemia. In these segments, there is a match between low blood flow and low function.
The precise pathogenesis of stunned and hibernating myocardium remains somewhat controversial and is beyond the scope of this article. However, it is critically important to recognize that both stunned and hibernating myocardial segments can regain function, and are, therefore, collectively referred to as viable myocardium. 1
Accurate identification of viable myocardial segments is critical in deciding whether to pursue medical or surgical treatment for left ventricular dysfunction caused by coronary artery disease. Accurate preoperative assessment of myocardial viability will predict which patients will regain left ventricular function and which will not. Clearly, patients with little or no viable myocardium should not be subjected to the risks of invasive surgery. Studies have shown that preoperative myocardial viability is an important predictor of clinical outcome after surgery. In a 1997 study, Pagley et al 2 showed that the extent of preoperative myocardial viability was the best indicator of 3-year cardiac-event-free survival after revascularization. Additionally, studies have suggested that revascularization of patients with viable myocardium may improve outcome independent of whether viable segments regain function postoperatively, although the mechanism by which this occurs remains controversial. 3 Management of patients with coronary artery disease, therefore, depends on precise determination of myocardial viability.
Imaging modalities for assessment of myocardial viability
There are essentially 4 methods of assessing myocardial viability in clinical practice today. Single-photon emission computed tomography (SPECT) with 201 thallium (201 Tl) has been widely utilized for this purpose. 201 Tl functions as a potassium analogue that is actively transported into myocytes via the Na + /K + ATPase located on the myocyte cell membrane. Initial images after injection indicate areas of decreased perfusion, while images obtained after a delay or redistribution phase show that these areas are filled in, revealing myocardium that retains viability despite diminished initial perfusion. This technique offers the benefit of being fairly easy to perform with widely available equipment. However, a meta-analysis has shown that it can overestimate viability with a specificity of 49%, although the sensitivity is high (88%). 4
Stress echocardiography (either with low-dose dobutamine or dipyramidole) has also been widely used to assess myocardial viability. Infusion of the "stress" agent, either a direct inotrope or a vasodilator, causes previously dysfunctional but viable myocardium to resume contractile function. This is referred to as stress-induced contractile reserve. The same meta-analysis referred to above reported a sensitivity of 84% and a specificity of 81% with this technique. 4 However, it is important to note that the sensitivity and specificity of echocardiography decreases with increasing left ventricular dysfunction--precisely the population that stands to benefit most from myocardial viability assessment. 5 Advantages of echocardiography include portability, speed, and its relative low cost; however, it is commonly limited by poor acoustic windows and is interpreted subjectively, often with high interobserver variability. 1
The gold standard for assessing in vivo myocardial viability has been functional imaging with fluorodeoxyglucose (FDG) PET. This technique involves comparing myocardial perfusion using a perfusion agent with functional metabolic images obtained with FDG. Myocardial segments that indicate normal or decreased perfusion but maintain metabolism are classified as viable. Because PET is the gold standard, MRI techniques are judged comparatively and have been shown to be approximately 95% concordant. 6 While generally agreed to be a quite accurate technique, PET imaging requires equipment and radiotracers that may not be readily available. Additionally, as currently practiced, it is a comparatively low-resolution study with pixel size of 8 mm, and it does not routinely provide functional information regarding wall motion and ejection fraction. Examination time for the patient is long, requiring several hours for a complete perfusion and metabolic study.
Delayed contrast-enhanced MRI
As opposed to nuclear methods, viability assessment by MRI is a nonstress examination that provides high-resolution detail, including functional assessment of the left ventricle in approximately 30 minutes. Assessment of myocardial viability is performed using 5- to 20-minute delayed, gadolinium-enhanced MRI. On delayed MRI, there is a relatively decreased washout of the gadolinium contrast agent in areas of myocardium that have been replaced by fibrosis or scar. In normal viable myocardium, the gadolinium contrast agent washes out more rapidly than it does from the fibrosis or scar. Since the difference between normal and abnormal myocardium is based on washout kinetics, images that are delayed by 5 to 20 minutes after contrast injection will optimally depict the fibrosis or scar.
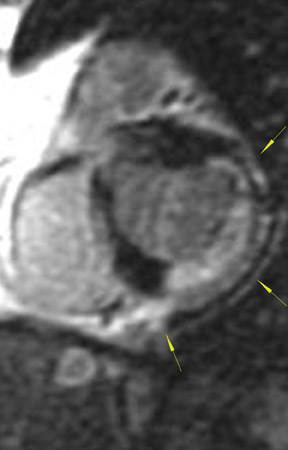
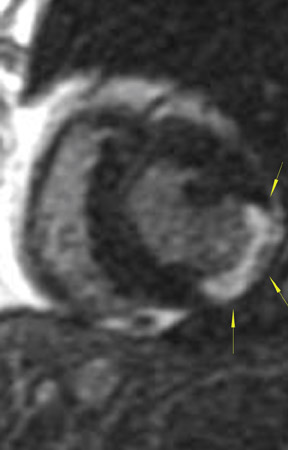
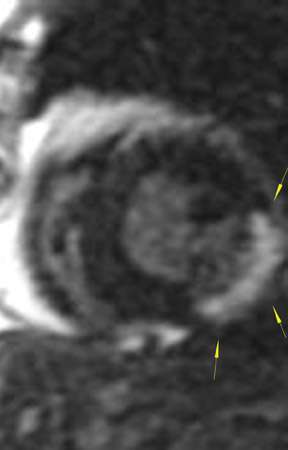
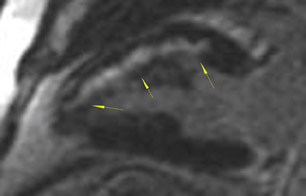
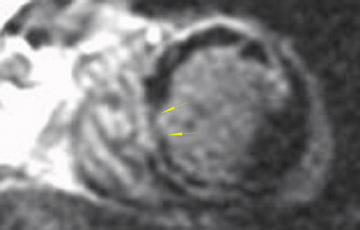
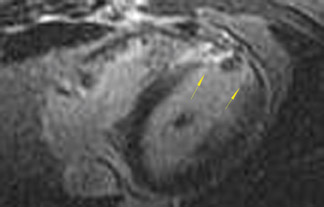
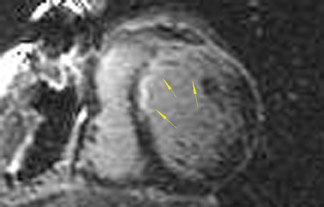
The differences in gadolinium enhancement on MRI of viable myocardium and fibrosis or scar have been known for many years. Recently, however, MRI pulse sequences have been developed that greatly improve the conspicuity of the enhanced areas of myocardium that have been replaced by fibrosis or scar. The pulse sequence used is an inversion-recovery prepared gradient-echo sequence. 7 In this method, an inversion pulse is used to the signal from normal myocardium. Myocardium that is replaced by fibrosis or scar retains gadolinium and shows very high signal intensity compared with the suppressed, darker myocardium (Figures 1 through 3).
This relationship of high signal on delayed gadolinium-enhanced MRI to fibrous or scar tissue has been repeatedly proven, and its accuracy has been confirmed. In a canine model, Kim et al 8 reported that infarcts exhibited hyperenhancement whether acute or chronic, whereas stunned or hibernating myocardium did not. Additionally, they showed that the extent of myocardial hyperenhancement matched the histologic extent of myocyte necrosis.
In human trials, delayed contrast-enhanced MRI was found to be an accurate method for determining myocardial viability in which recovery of myocardial function after coronary bypass surgery was the reference standard. In 52 patients who underwent coronary revascularization, investigators identified improved regional function in 82% of segments with no preoperative hyperenhancement, 64% of segments with 1% to 25% hyperenhancement, and 37% of segments with 26% to 50% hyperenhancement. 9 Indeed, studies have shown that the ability of MRI to predict restoration of myocardial function after revascularization is actually best in segments with the most severe initial dysfunction--exactly the opposite of stress echocardiography. When investigators considered only segments with severe preoperative dysfunction, the positive and negative predictive values for the recovery of function were 81% and 72%, respectively. 9
The high spatial resolution of MRI allows for a more accurate determination of the transmural extent of viability as opposed to a binary classification of a myocardial wall segment as either viable or not, as determined by nuclear medicine techniques and echocardiography. 10 This distinction is critical because it has been repeatedly shown that revascularization of patients with viable myocardium leads to significant improvement in clinical outcome.
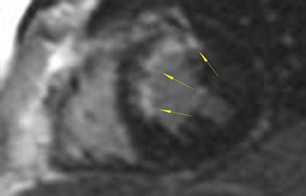
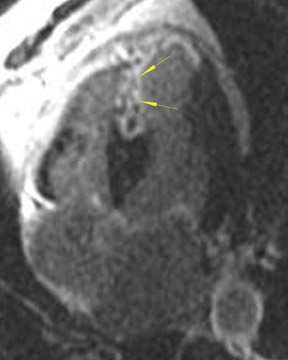
As an example of the use of MRI for viability imaging, surgical ventricular reconstruction (the "Dor procedure") is being performed in patients with congestive heart failure and a left ventricular aneursym. In this procedure, a left ventriculotomy is performed, and the ventricular aneurysm is reduced in size by exclusion of the scar from the endocardial volume of the left ventricle. The goal of the cardiac surgeon is to incise only that part of the ventricle that is fibrosis or scar; myocardium that is viable may recover function from coronary artery bypass alone. Viability MRI is used to determine 1) the site of the fibrosis or scar, 2) whether it is transmural or subendocardial, and 3) the extent and coronary artery territory of the fibrosis or scar (Figure 4). In addition, incidental findings (such as left ventricular thrombus, mitral valve regurgitation, and left ventricular volumes) are routinely assessed with MRI in the same setting.
Other applications
Delayed enhancement of the myocardium is not specific for prior fibrosis or scar from myocardial infarction. Prior myocarditis or fibrosis in association with hypertrophic cardiomyopathy, for example, also exhibits delayed enhancement when the same MRI technique is used. In addition, acute inflammation, tumors, and granulomatous disease show delayed enhancement. Recently, delayed enhancement has been shown to be found in arrhythmogenic right ventricular dysplasia. 11 Although the patterns of enhancement of the myocardium usually differ in these conditions when compared with enhancement from prior myocardial infarction, the correct diagnosis requires knowledge of the clinical context of the MRI examination.
Conclusion
The ease of use and interpretation of MRI for myocardial viability assessment has resulted in rapid growth in the use of this technique. As such, the method has become one of the most important cardiac imaging tests and a clinical gold standard for identification of fibrosis or scar in the ventricle. In a single examination, MRI provides the critical anatomic, functional, and viability information required by the clinician to optimize patient management.