MicroStent Vascular Stent Receives FDA Breakthrough Device Designation
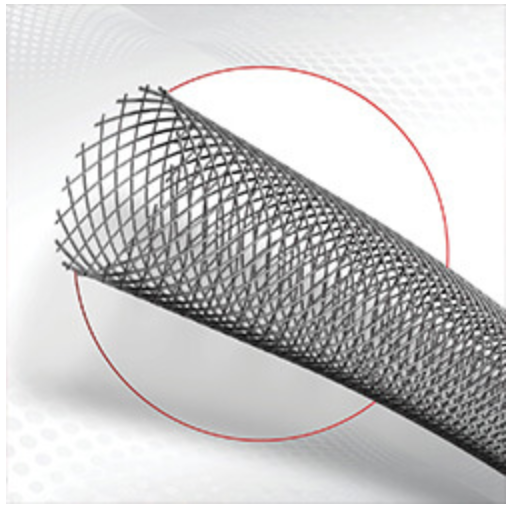 Micro Medical Solutions (MMS) announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its MicroStent vascular stent. This novel technology is designed to achieve and maintain vessel patency, enhance wound treatment, and improve quality of life and blood flow in order to reduce amputation and mortality for patients with CLTI resulting from peripheral artery disease (PAD).
Micro Medical Solutions (MMS) announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its MicroStent vascular stent. This novel technology is designed to achieve and maintain vessel patency, enhance wound treatment, and improve quality of life and blood flow in order to reduce amputation and mortality for patients with CLTI resulting from peripheral artery disease (PAD).
FDA Breakthrough Device Designation is reserved for technologies that demonstrate improved effectiveness compared to the standard of care in the treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The goal is to give patients and doctors timely access to these medical devices by speeding up their development, assessment, and review, while preserving the statutory standards for premarket approval. To further enhance patients' access to essential new medical technologies, a Medicare coverage pathway called Medicare Coverage of Innovative Technology ensures FDA Breakthrough Devices are covered for four years.
"FDA Breakthrough Device Designation is an exciting step forward for MMS and for patients with CLTI, who will have expedited access to MicroStent. We are pleased that the FDA is recognizing the importance and severity of CLTI and look forward to collaborating with them as we go through the PMA process," said Micro Medical Solutions CEO Gregory Sullivan. "As we remain focused on the completion of our FDA clinical study, STAND, it is gratifying to know we are now one step closer to our goal to help as many CLTI patients as possible live without the trauma of amputation."
MMS is currently engaged in an FDA randomized, multicenter pivotal clinical study for MicroStent, called STAND (A Clinical Evaluation of the MicroSTent PeripherAl Vascular SteNt in subjects with Arterial Disease Below the Knee), which began in May 2020 and will continue at up to 25 sites across the U.S. In addition, the MMS study HEAL (An All-Comers Observational Study of the MicroStent Peripheral Vascular Stent System in Subjects With Peripheral Arterial Disease) is currently enrolling patients at centers in the EU.