Klippel-Trenaunay Syndrome
Case Summary
An infant presented with a large port-wine stain of the right lower extremity, pelvis, and trunk that was present at birth. Additionally, the right lower extremity was larger than the left and there was intermittent bleeding from skin lesions of the right lower extremity. A punch biopsy of a skin lesion was performed for genetic testing, documenting a somatic, pathogenic PIK3CA variant. The diagnosis was PIK3CA-related overgrowth spectrum, and the phenotype was consistent with Klippel-Trenaunay syndrome (KTS).
Imaging Findings
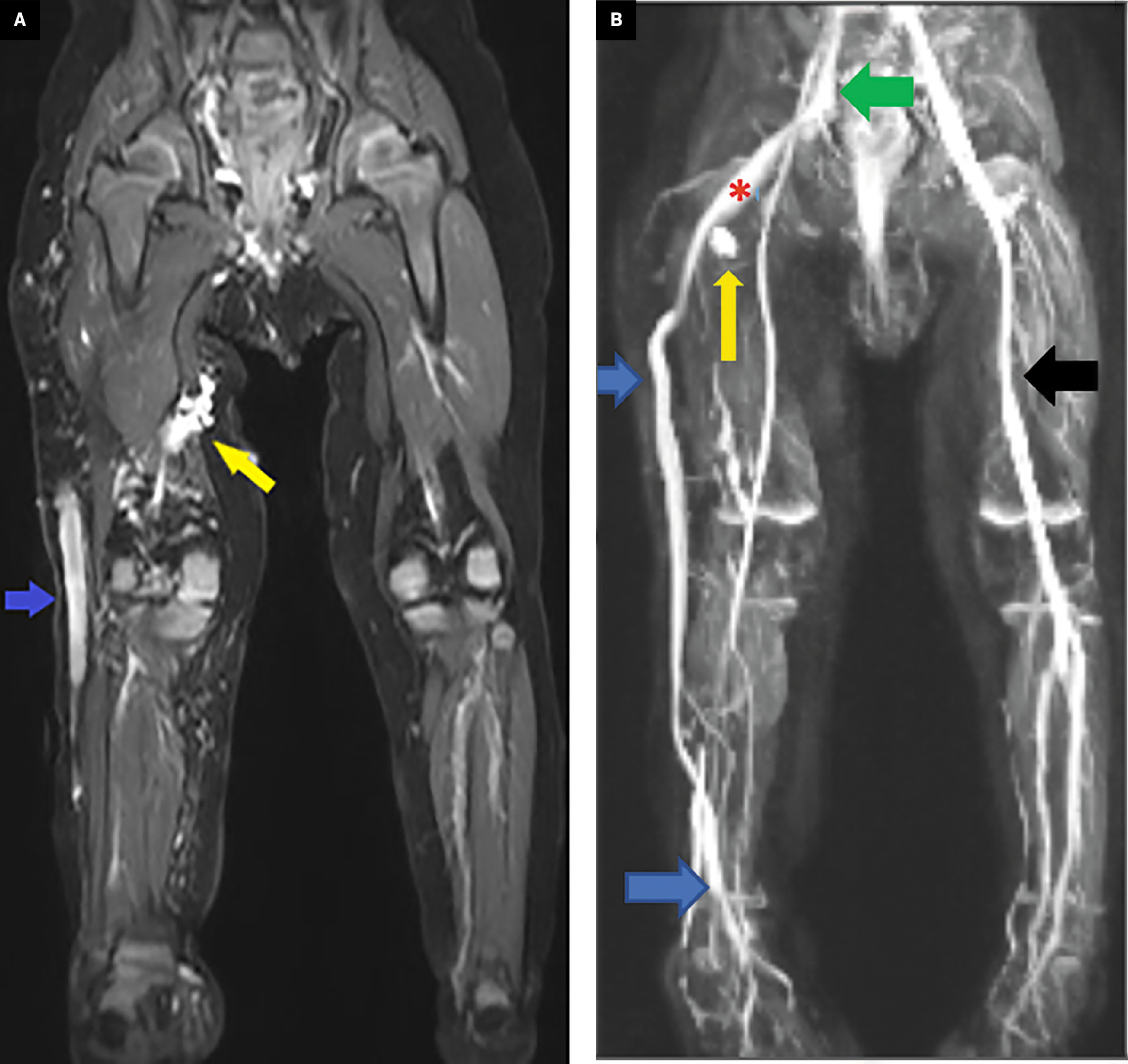
MRI of the lower extremities and pelvis showed the abnormalities ( Figure 1 ), including a large lateral marginal vein extending from the foot to the proximal thigh, which drains centrally. Additionally, there were scattered lymphatic and venous malformations in the right lower extremity.
MRI (A, B). Coronal STIR (A) and MR venography (B) of the lower extremities and pelvis. A large lateral marginal vein extends from the foot to the proximal thigh (blue arrow), where it continues as the sciatic vein (red asterisk) and then drains into the right internal iliac vein (green arrow). The lateral marginal and sciatic veins are persistent embryonic veins. The right lower-extremity deep veins are hypoplastic. Note the normal left femoral vein (black arrow). Additionally, scattered lymphatic and venous malformations are shown in the right lower extremity (yellow arrows).
Diagnosis
Klippel-Trenauney syndrome (KTS)
Differential Diagnosis
CLOVES Syndrome
The limb overgrowth and port wine stains seen in KTS manifest similarly to CLOVES (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/skeletal/spinal/anomalies) syndrome, another manifestation of PIK3CA gene mutations. In CLOVES, truncal involvement with vascular malformations and fatty overgrowth is more common. Additionally, tethered spinal cord, scoliosis, and renal abnormalities such as Wilms tumor are associated with CLOVES syndrome but are not typically seen in KTS.
Proteus Syndrome
Proteus syndrome results from a mutation in the AKT1 gene pathway, resulting in an asymmetric overgrowth pattern that can affect all body parts. When unilateral, this may appear similarly to the overgrowth seen in KTS, but Proteus syndrome presents much more frequently with progressive skeletal abnormalities and pulmonary disease.
Maffucci Syndrome
Maffucci syndrome is due to an IDH1 gene mutation and initially presents with enchondromas in the long bones of the arms and legs. Vascular malformations associated with this syndrome are progressive but usually present at 5 years of age or older. The primary risk of this syndrome is the development of chondrosarcoma.
Sturge-Weber Syndrome
Sturge-Weber syndrome is another genetic disorder that results from a mutation of the GNAQ gene and is associated with facial port-wine stains and vascular malformations, although the malformations are almost always seen in the brain. Additionally, these patients often have delays in cognitive development and ophthalmologic abnormalities, which are not seen in isolated KTS.
Discussion
The diagnosis of KTS is usually clinical. The initial diagnostic imaging modality of choice is often Doppler US, which allows for visualization of the varicosities as well as appreciation of any concurrent thrombosis or venous insufficiency. The extent of the US findings directly relates to disease severity. Once extensive venous thrombosis is noted in the setting of possible KTS or a therapeutic intervention is considered, CT or MRI is often needed to determine disease extent. Additionally, these imaging modalities can evaluate for other malformations, including spinal cavernous malformations, pulmonary anomalies, and gastrointestinal malformations.1
KTS is an uncommon pediatric disease characterized by an activating mutation in the PIK3CA gene, resulting in global or focal tissue overgrowth. The disease is a common subtype of PIK3CA-related overgrowth syndromes and has a variable but classic presentation due to the mosaic pattern of gene mutation.1 KTS is also associated with a triad of cutaneous angiomas, venous and lymphatic abnormalities, and soft-tissue or bony hypertrophy. A diagnosis of KTS requires 2 of these 3 features.2, 3 These abnormalities have various clinical consequences, predominantly limb pain, fatigue, and recurrent superficial and deep-vein thrombosis.4 On examination, children with KTS have overgrowth of the affected limbs and/or cutaneous malformations on the trunk. Port-wine stains are the most common skin finding, which are cutaneous manifestations of the underlying capillary malformations.
The focus of therapy in refractory KTS is obliteration or removal of the persistent embryonic veins, which are the common cause of signs and symptoms. The 2 vessels that constitute the most problematic veins in the leg are the lateral marginal vein (LMV) and the persistent sciatic vein (PSV).5 The LMV is an embryonic vessel that originates on the dorsal side of the foot and eventually contributes to the small saphenous vein and superficial venous system before naturally regressing. The PSV is a deep vein of the posterior thigh considered the major tributary of the developing deep-venous system before regressing. When these vessels persist, they disrupt the physiological growth of normal venous anatomy and often dilate to large diameters, greatly increasing the risk of venous stasis. When severe, this can result in life-threatening complications, including large-volume bleeding, pulmonary emboli, and venous thromboses.2 Despite being a common in affected children, the prevalence of these marginal veins is conservatively measured at around 15% to 20%, likely due to the highly variable phenotype of KTS.5
When these embryonic veins only cause mild and nondisruptive symptoms, conservative treatment with compression stockings, pain control, and avoidance of injury are the first-line treatment. Traditionally, surgery has been the primary treatment method for severe or refractory cases. The surgical approach depends on disease extent and usually includes vein stripping, stab phlebectomies (whereby the vein is hooked through a small excision, brought outside the skin, and removed), and gross excision.
Surgery has largely been replaced by more minimally invasive techniques, including embolization using coils, vascular plugs and other materials, endovenous laser ablation therapy (EVLT) such as radiofrequency ablation (RFA), and sclerotherapy. These methods occlude abnormal veins and avoid the potential surgical complications of poor wound closure and persistent bleeding, and are first-line therapies for persistent KTS.1 Other ablative techniques, such as cryoablation, cannot currently deliver therapy to the entire embryonic vessel, which could result in collateralization and incomplete response. The best approach is not yet known.
EVLT can be used to treat persistent embryonic veins, and acts by emitting a wavelength of light that generates photon-induced damage to the vessel wall as well as thermal damage to the surrounding area.6 EVLT is generally well-tolerated, with complications including ecchymosis, skin burns, and thrombophlebitis along the ablated vein. Rarely, heat-induced thrombus may occur, which is defined as postprocedural thrombus propagating into deeper vessels cephalad to the treated area.7 RFA may cause tissue necrosis in a localized area by agitating adjacent molecules and producing high temperatures.7 The complications of RFA are the same as those of EVLT, owing to the similar method of heat-induced vascular damage.8
Sclerotherapy can treat a variety of vascular diseases, including the persistent embryonic veins in KTS.9 In sclerotherapy, catheter injection of a sclerosant, either alone or in combination with other minimally invasive techniques, damages the intimal wall of the vessel and induces thrombosis. A variety of agents can be used based on radiologist preference. Modern agents include polidocanol and sodium tetradecyl sulfate, which thrombose the target vessel while avoiding the local destruction and ischemia that can occur with the high doses of ethanol needed to treat large vessels.10 Complications of sclerotherapy, such as deep-vein thrombosis and extension of thrombosis past the target lesion into a deeper venous system, can occur. More severe complications can result in both transient and permanent neurological deficits, including visual disturbance, migraine, stroke, and, rarely, death.6 The expected rate of neurological disturbance is less than 2%, with permanent damage even more rare.6
Conclusion
KTS is a disease that, when severe, has devastating clinical consequences, including severe bleeding, deep-vein thrombosis, and pulmonary emboli. Although the presentation of KTS is variable, the classic triad is cutaneous angiomas, venous and lymphatic abnormalities, and tissue overgrowth.. When KTS is suspected in a child with unilateral extremity overgrowth, imaging will help identify the problematic embryonic vein(s), both the LMV and the PSV. US will demonstrate a persistently dilated embryonic vein with thrombosis, and MRI can determine the extent of the lesion. MRI will additionally characterize the soft tissue and bony overgrowth and allow for treatment planning. Treatment for this syndrome has historically been surgical, which can be associated with significant complications. New minimally invasive techniques, including RFA, EVLT, and sclerotherapy, have expanded treatment options with fewer complications.
References
Related Articles
Citation
Maag BC, Towbin RB, Schaefer CM, Towbin AJ.Klippel-Trenaunay Syndrome. Appl Radiol. 2024; (5):10 - 13.
doi:10.37549/AR-D-24-0014
October 1, 2024