Effects of Gender-affirming Medical and Surgical Therapy on Breast Imaging Findings and Breast Cancer Risk
Images
Transgender individuals experience a self-concept of gender that differs from their sex assigned at birth.1 A transgender female self identifies as female and has been assigned male sex at birth (male-to-female). A transgender male self identifies as male and has been assigned female sex at birth (female-to-male). An estimated 1.3-2.0 million people in the United States identify as transgender; this is possibly underestimated given the lack of national census surveillance of gender identity.2,3 Some transgender individuals may choose to undergo gender-affirming hormone (GAH) therapy or gender-affirming surgery (GAS) to align their external appearance with their inner experience of gender.
This narrative aims to introduce readers to breast-related gender-affirmation therapy, review the most recent recommendations for breast cancer screening in the transgender population, and depict some of the expected breast imaging findings in the transgender population.
Culturally Competent Care
Owing to ongoing discrimination in the clinical setting, the transgender population experiences greater healthcare disparities than the cisgender population.4,5 Cisgender individuals identify their gender as concordant with their sex assigned at birth. Breast cancer screening rates for transgender patients are lower than those of cisgender women, potentially due to a lack of culturally competent providers.6
In a 2019 review of US breast care centers, including those on a list of the “Top 20,” none offered information regarding cancer screening or treatment in transgender individuals.7 In fact, most research on sexual minority women and cancer has focused on cisgender lesbians. Transgender patients can experience cancer care as “disorienting and uncoordinated” in relation to their gender-affirming care goals.8 Indeed, the belief that breast cancer is a “woman’s cancer” impacts how a transgender female experiences diagnosis and treatment.8
There are no standardized or evidence-based clinical practices on simultaneous GAH and medical cancer treatment, creating confusion for transgender females as they try to make informed medical decisions.8 A recent Canadian study reported that peer networking is a primary source of cancer information among transgender patients.8
Increasing screening rates in this population requires effort by radiologists and clinical staff to demonstrate cultural competency and create a welcoming environment. Intake forms should be amended to include gender identity, sex at birth, pronouns, preferred name, and history of medical or surgical gender-affirming therapy. Gender-inclusive signage, including gender-neutral bathrooms and changing rooms, should also be made available.
Referring physicians, radiologists, and clinical staff may benefit from transgender cultural competency training. Appropriate usage of gender-neutral language should be taught and implemented. If a provider is unsure of a patient’s pronouns, inquiring which pronouns the patient uses is a best practice.
Considerations in Transgender Females
Gender-affirming Therapy
The transgender female may choose a variety of gender-affirming medical or surgical interventions to align her physical appearance with her gender identity. Medical therapies include estradiol for feminization (exogenous estrogen), anti-androgens such as spironolactone, and gonadotropin- releasing hormone agonists (GnRH). At a histologic level, long-term estrogen therapy leads to breast tissue development by inducing metaplasia with the duct and lobule with acini formation. Breast tissue development is histologically similar to that in cisgender females.9, 10 The transgender female on GAH therapy will demonstrate a defined nipple-areolar complex. Pseudolactational changes demonstrating milk-like secretions have also been documented with GAH in this population.9, 10
Gynecomastia in the cisgender male breast is composed of ductal and stromal hyperplasia without lobule and acini formation.11 The term gynecomastia should not be used in reporting terminology for transgender females.
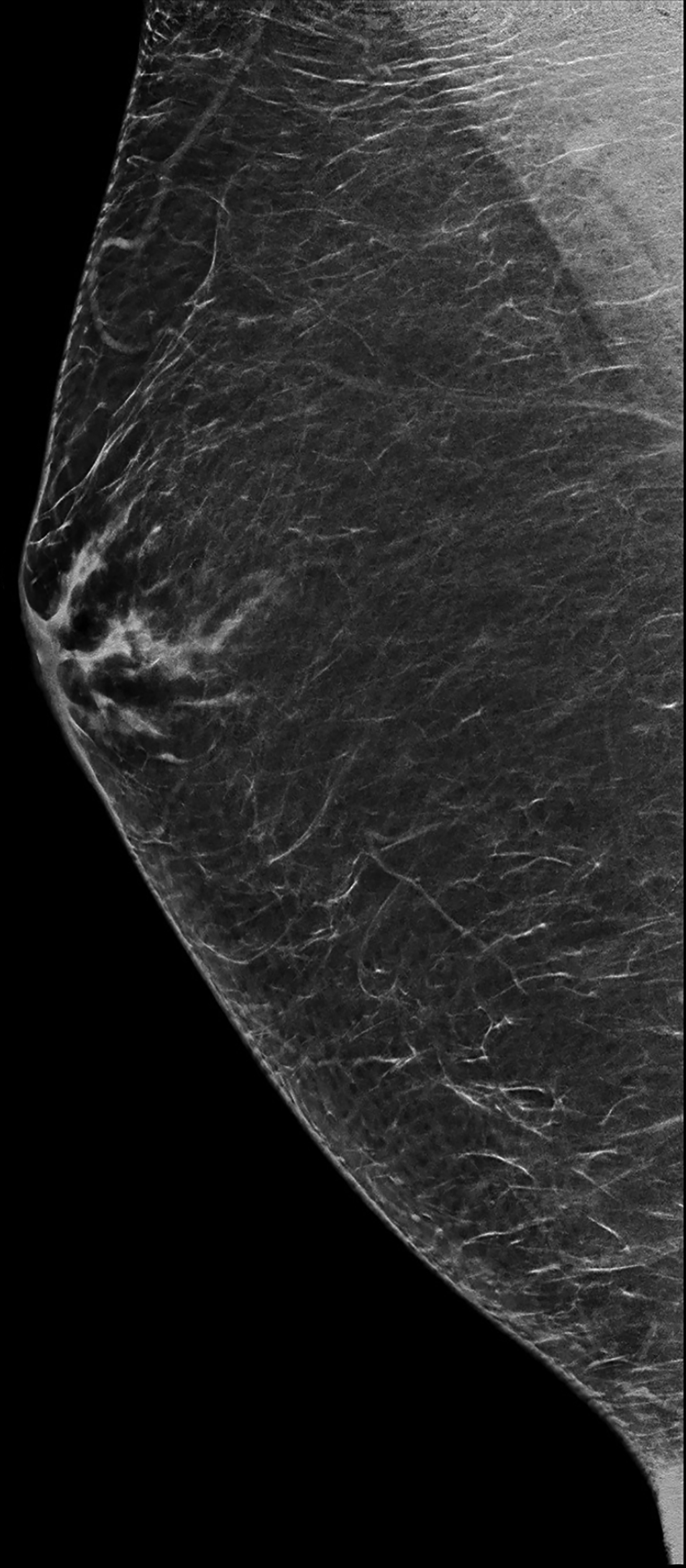
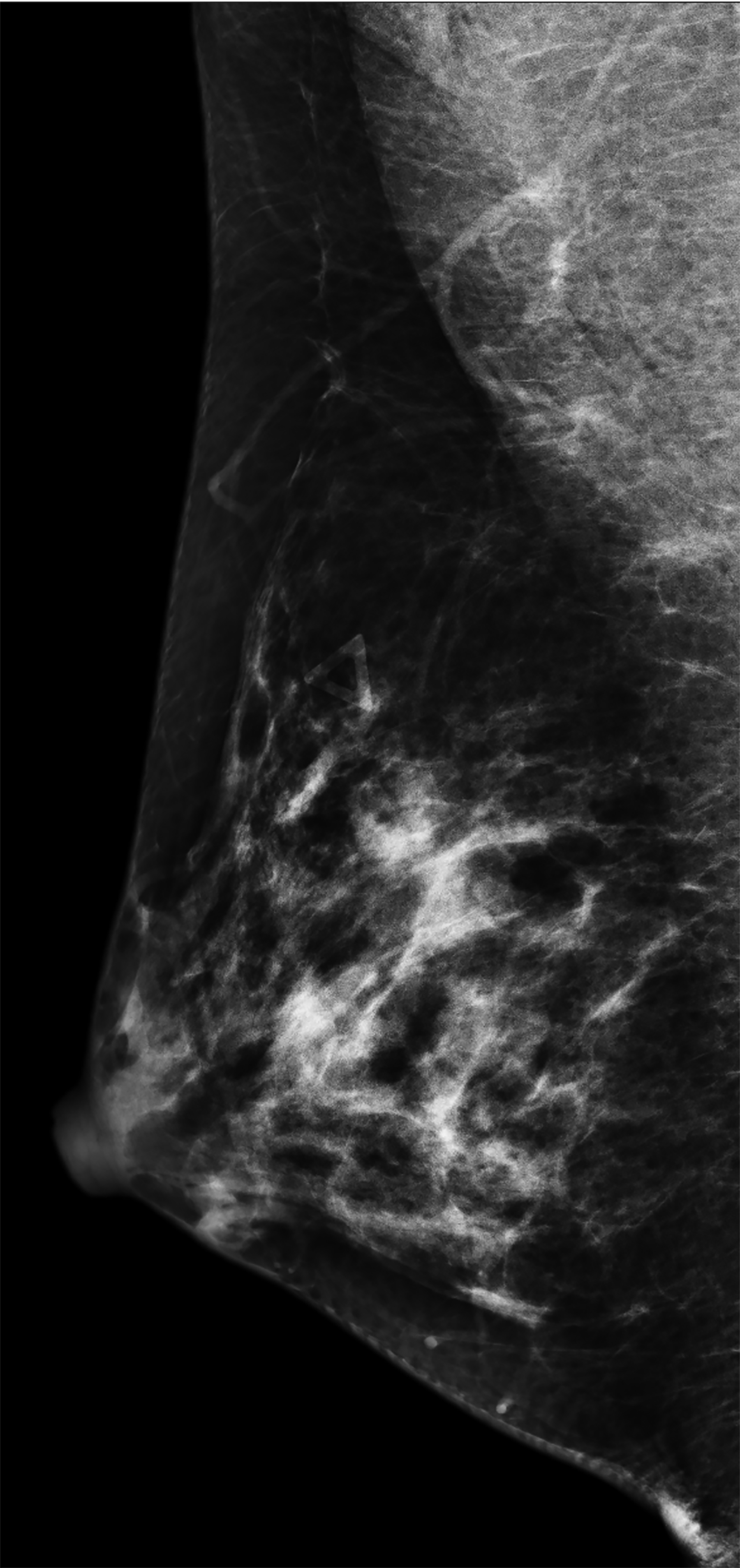
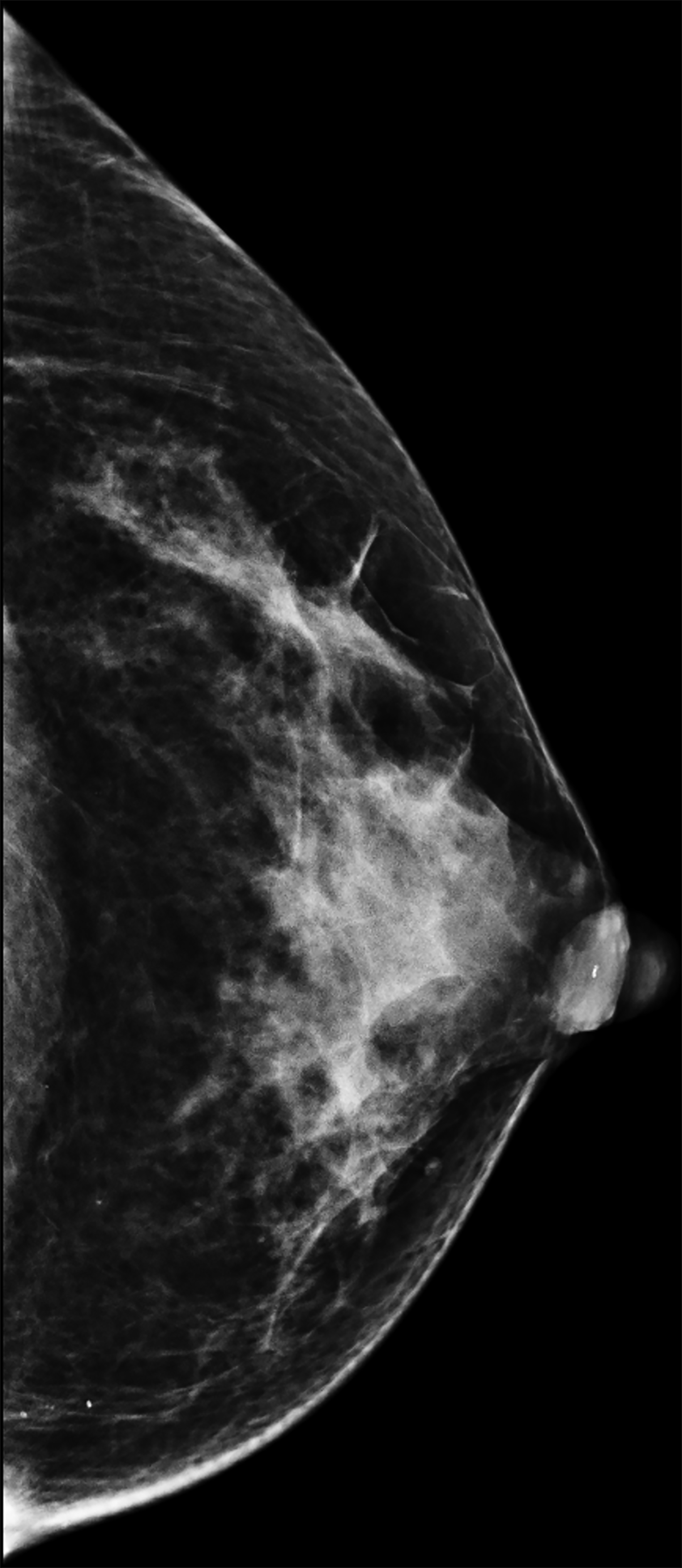
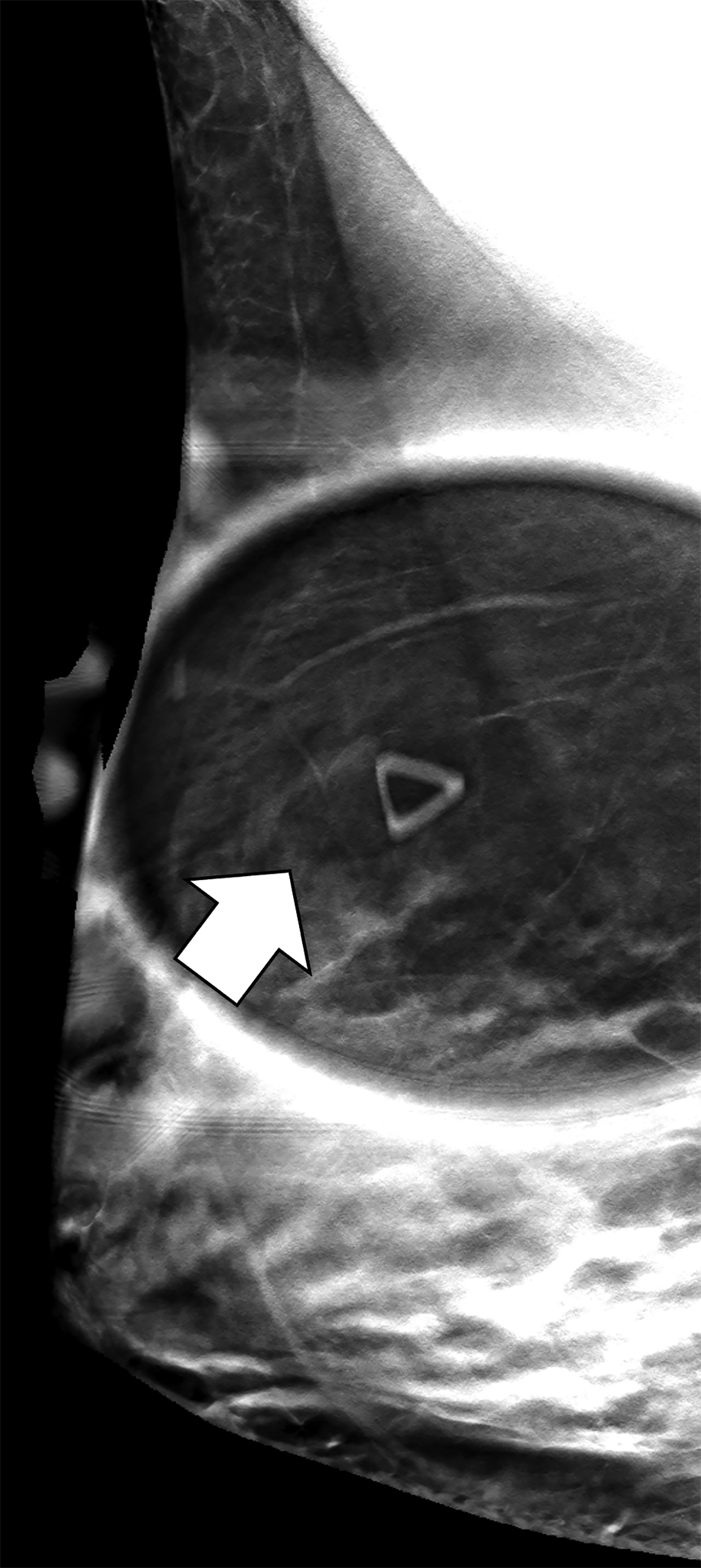
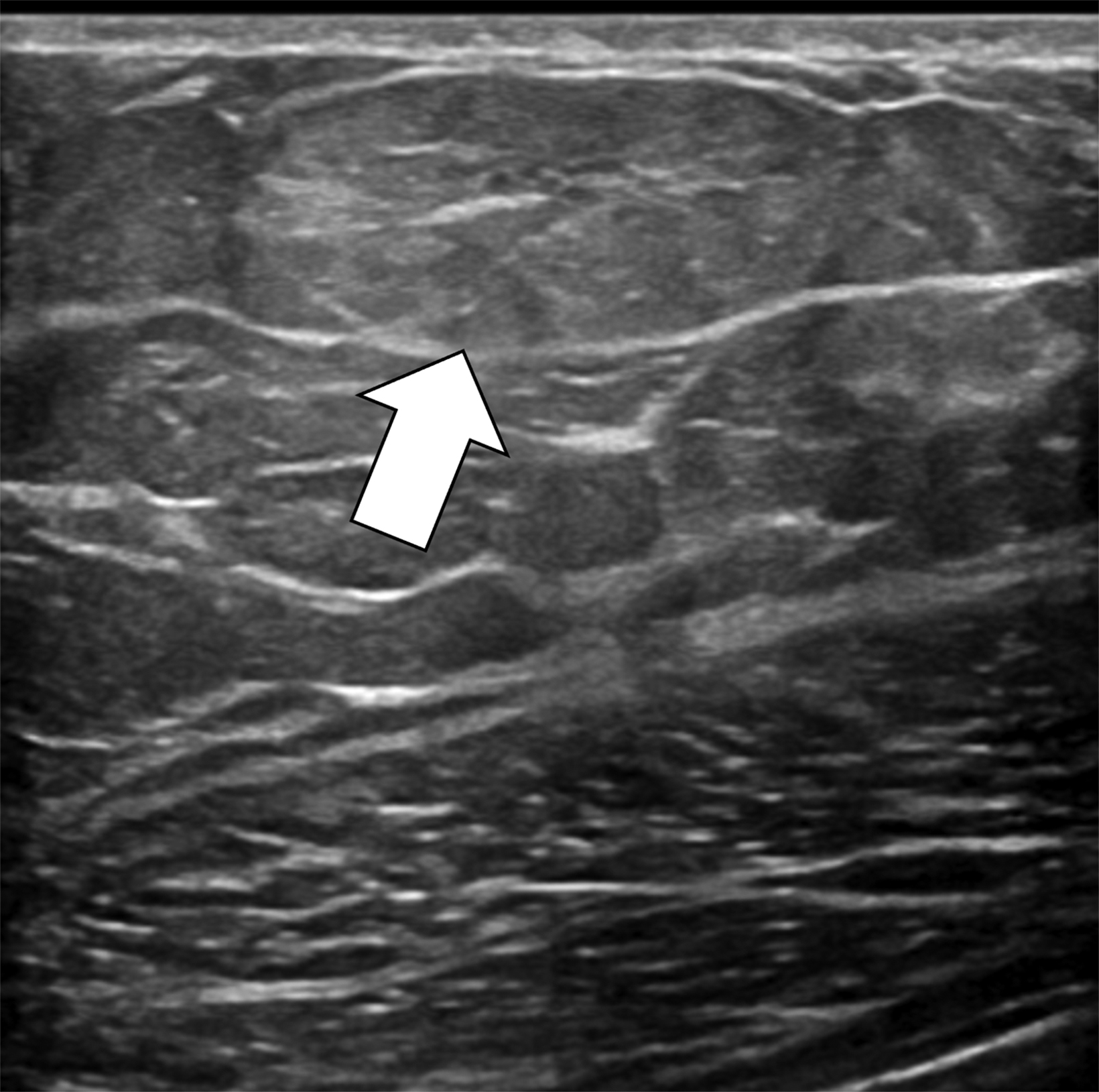
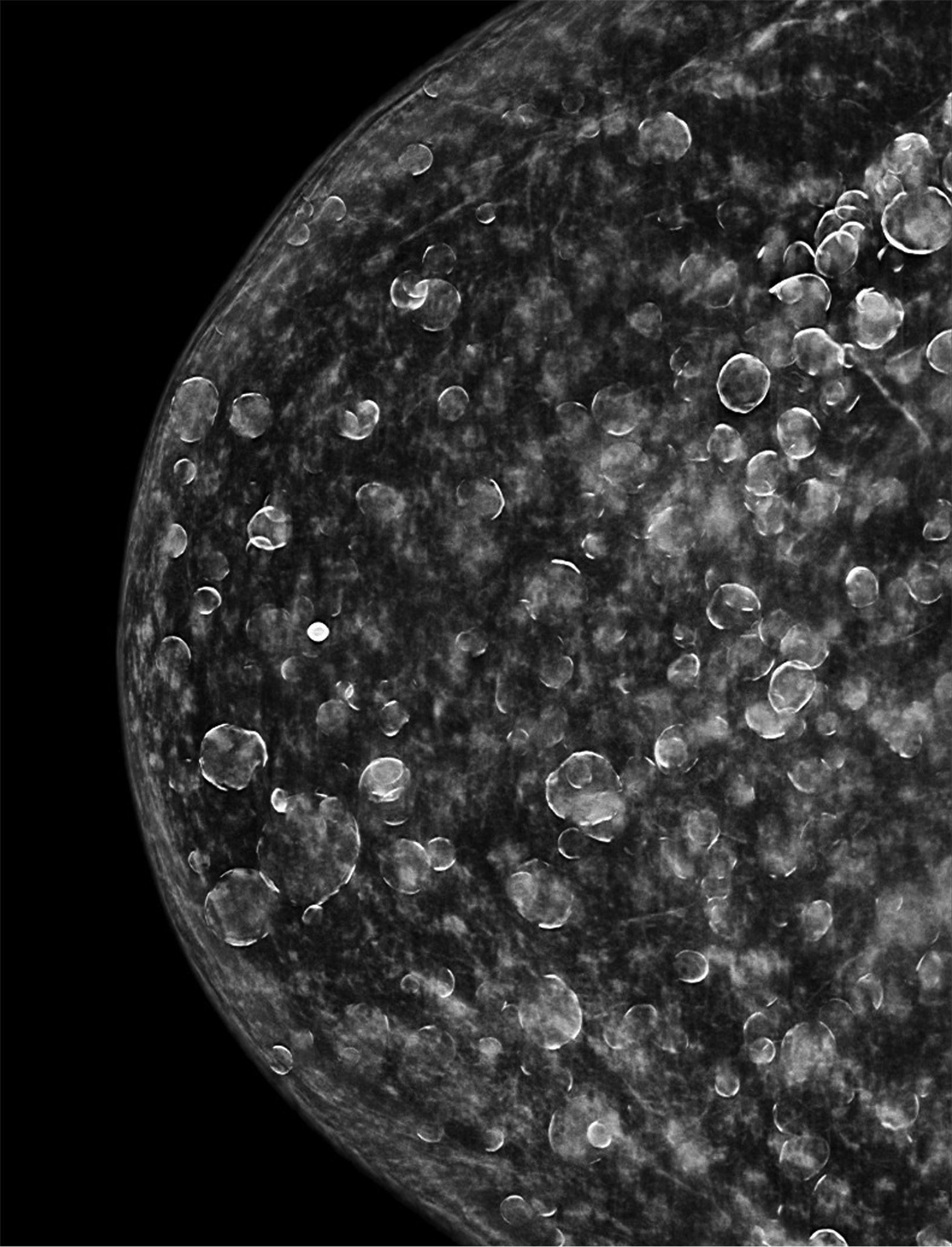
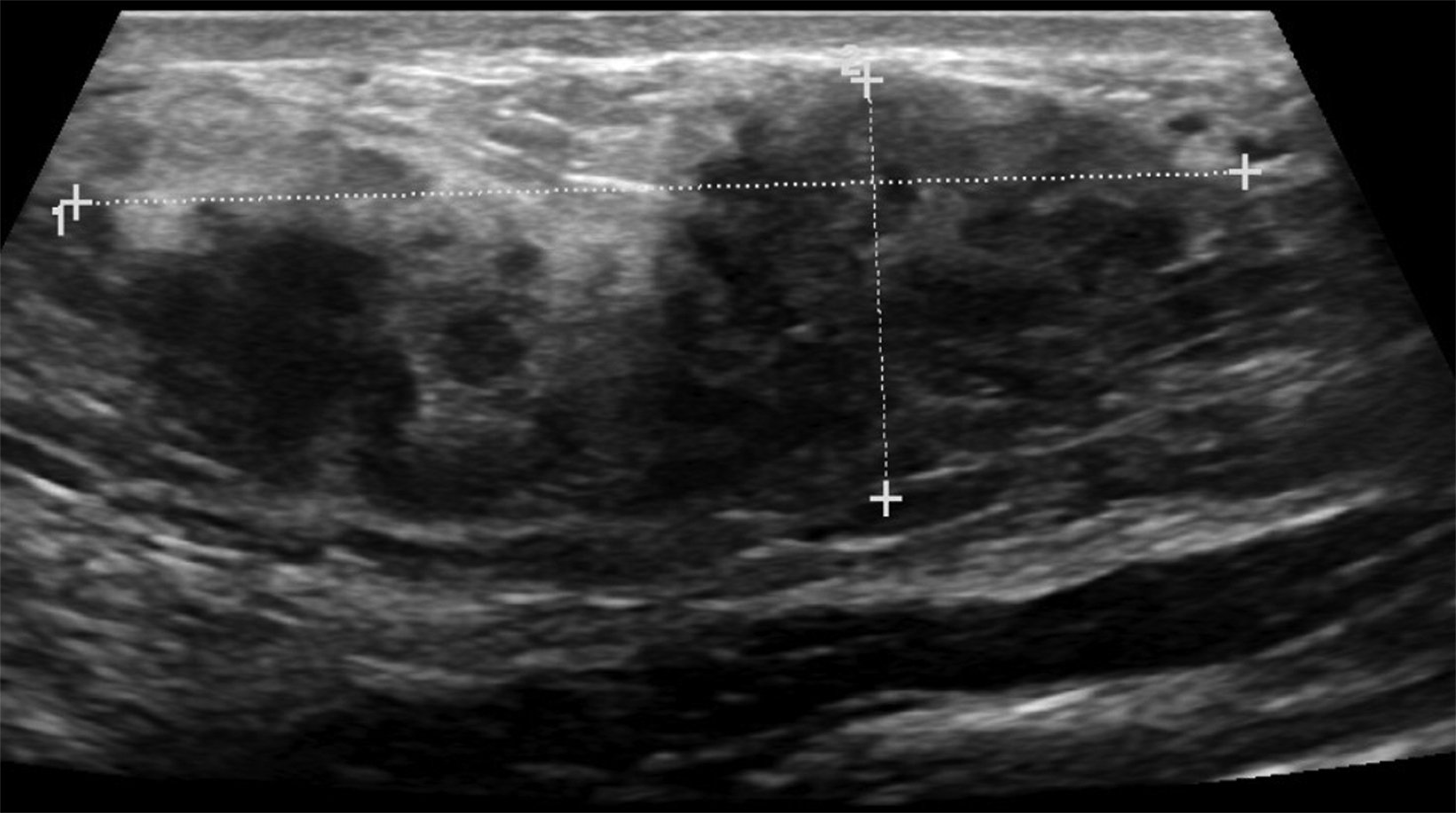
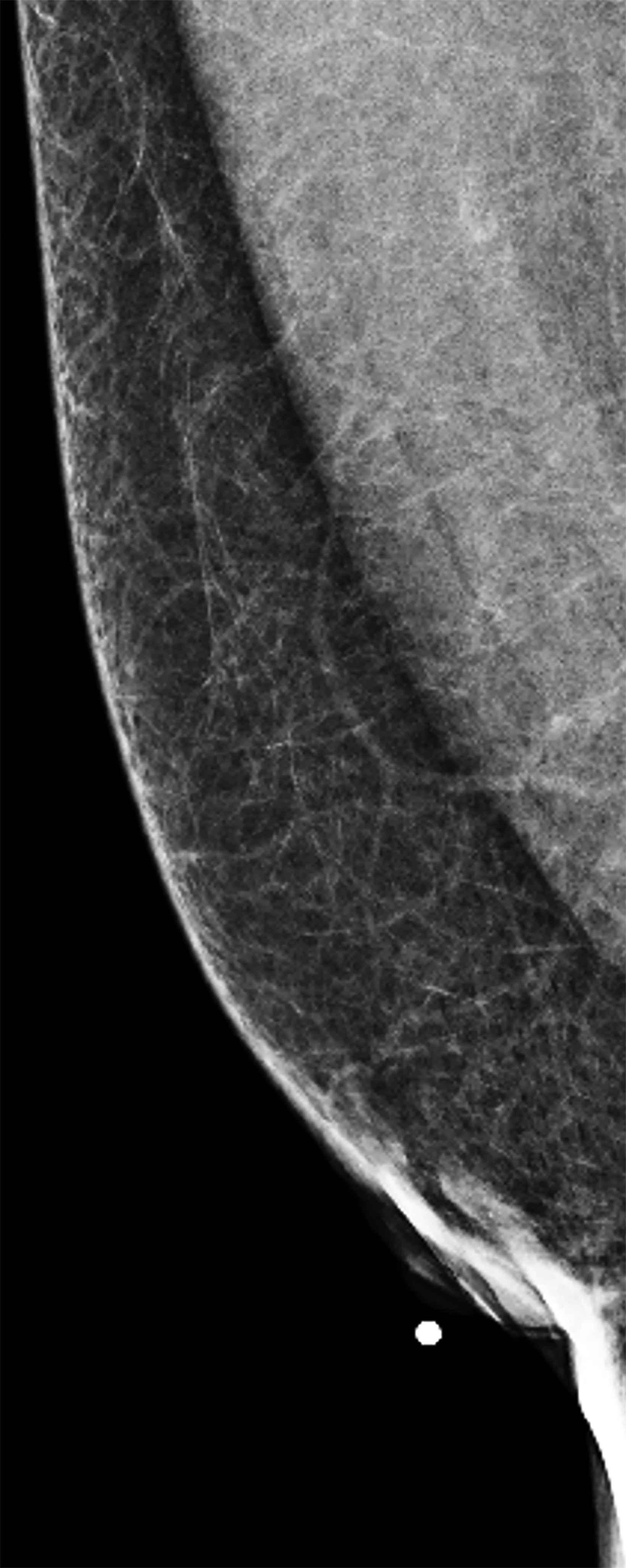
The transgender female breast undergoes muted Tanner stages of parenchymal development, with size and tissue density varying between individuals.9 Maximum tissue development is achieved after two or three years of GAH therapy (Figure 1).9, 11 Benign breast entities commonly encountered in cisgender females, such as fibroadenoma, lipoma, lymph node with follicular hyperplasia, flat epithelia atypia, and atypical ductal hyperplasia, are also observed in hormonally induced breast tissue of transgender females (Figures 2, 3).9
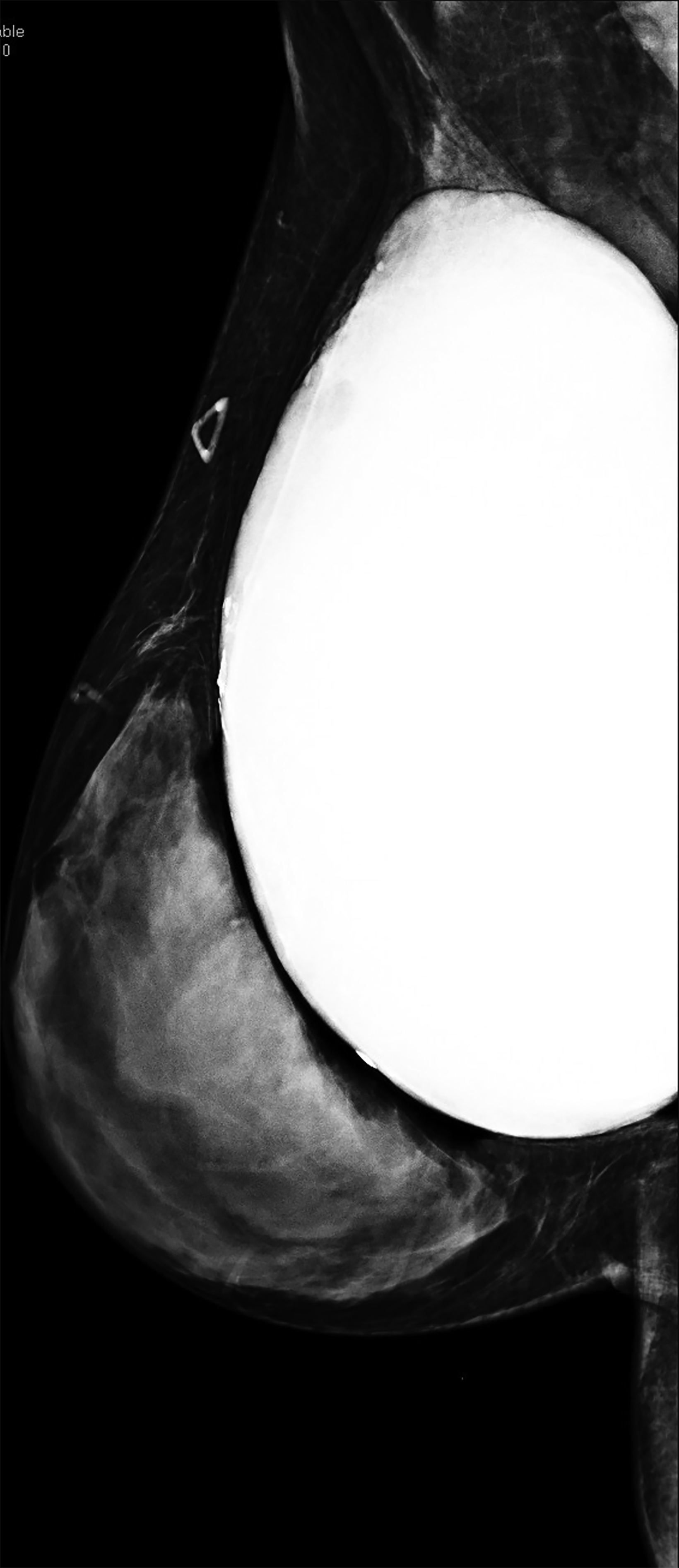
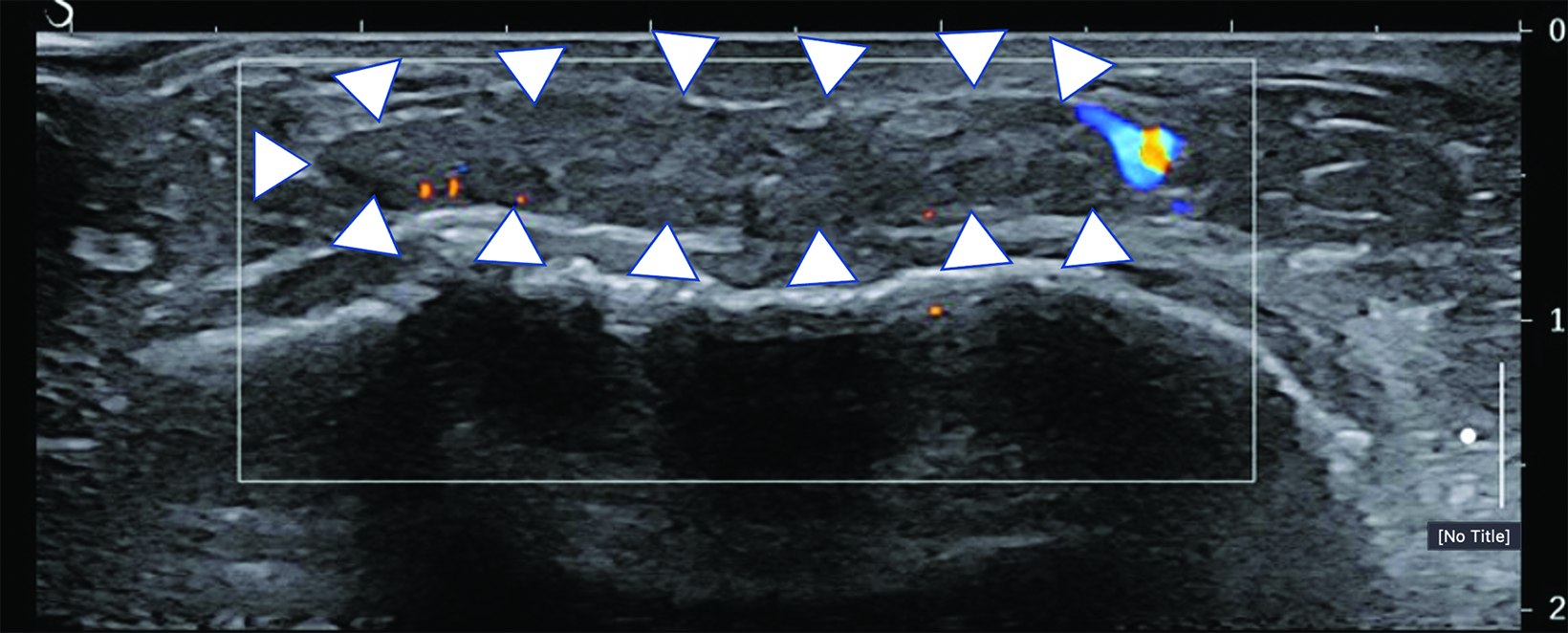
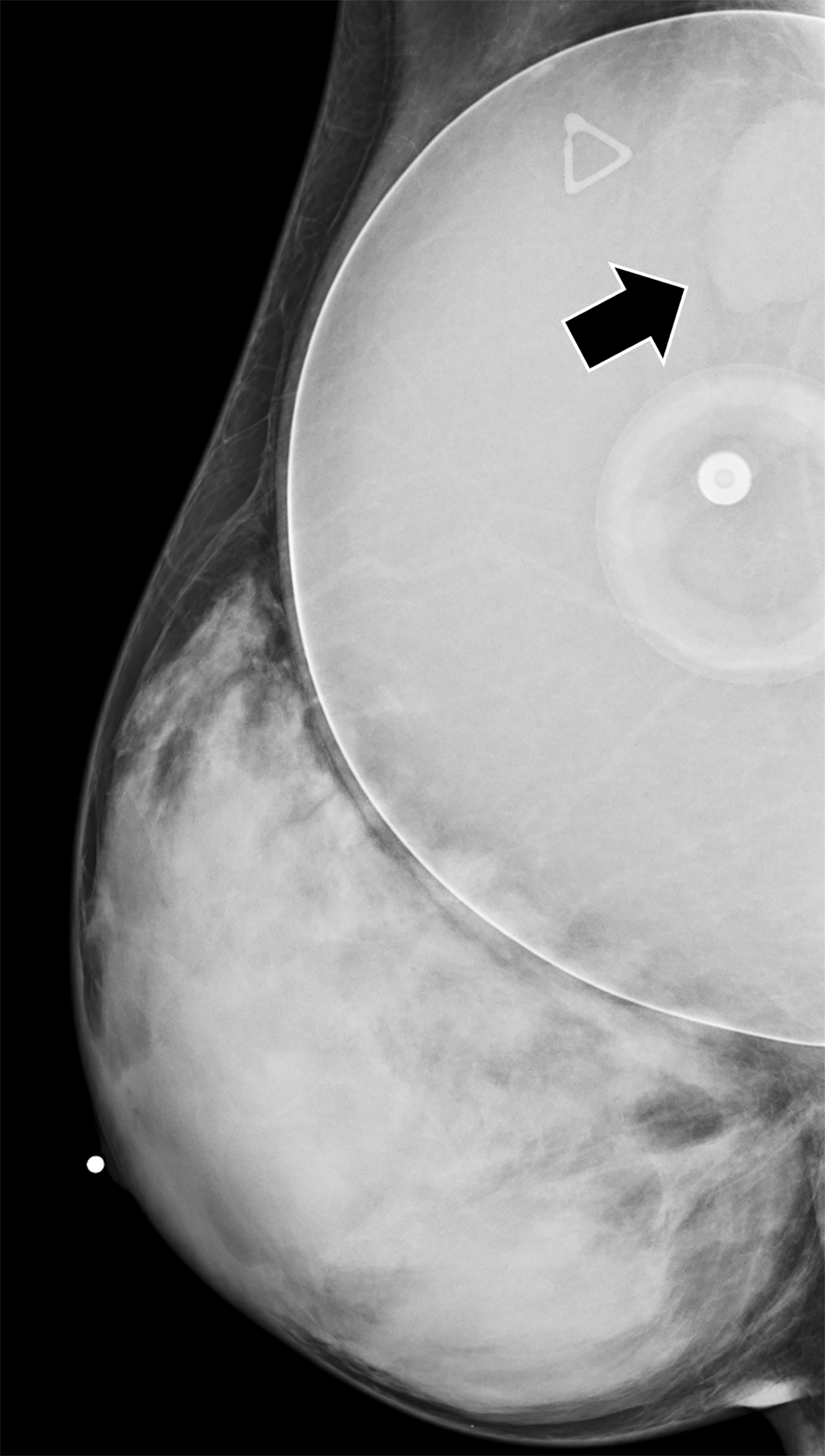
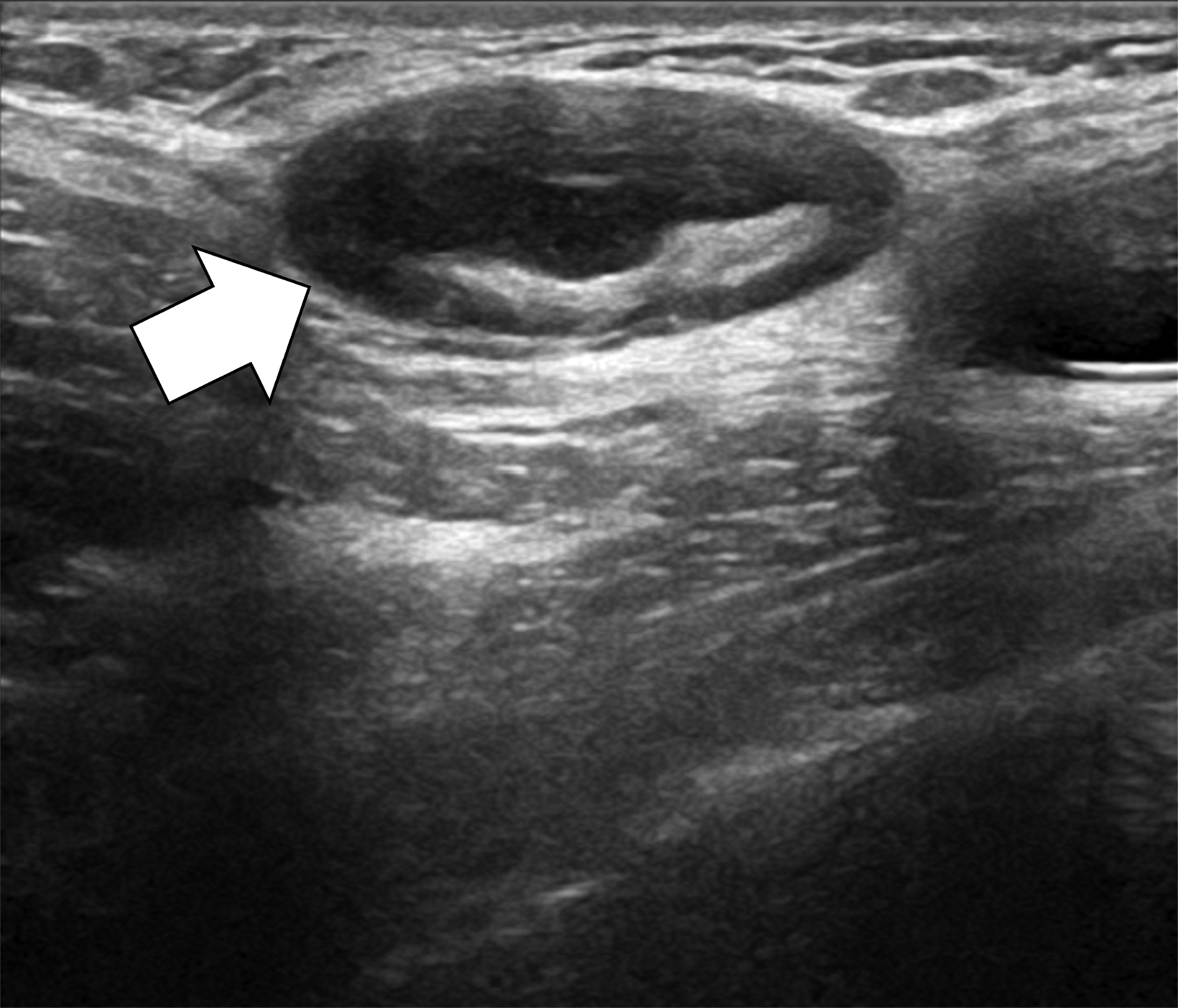
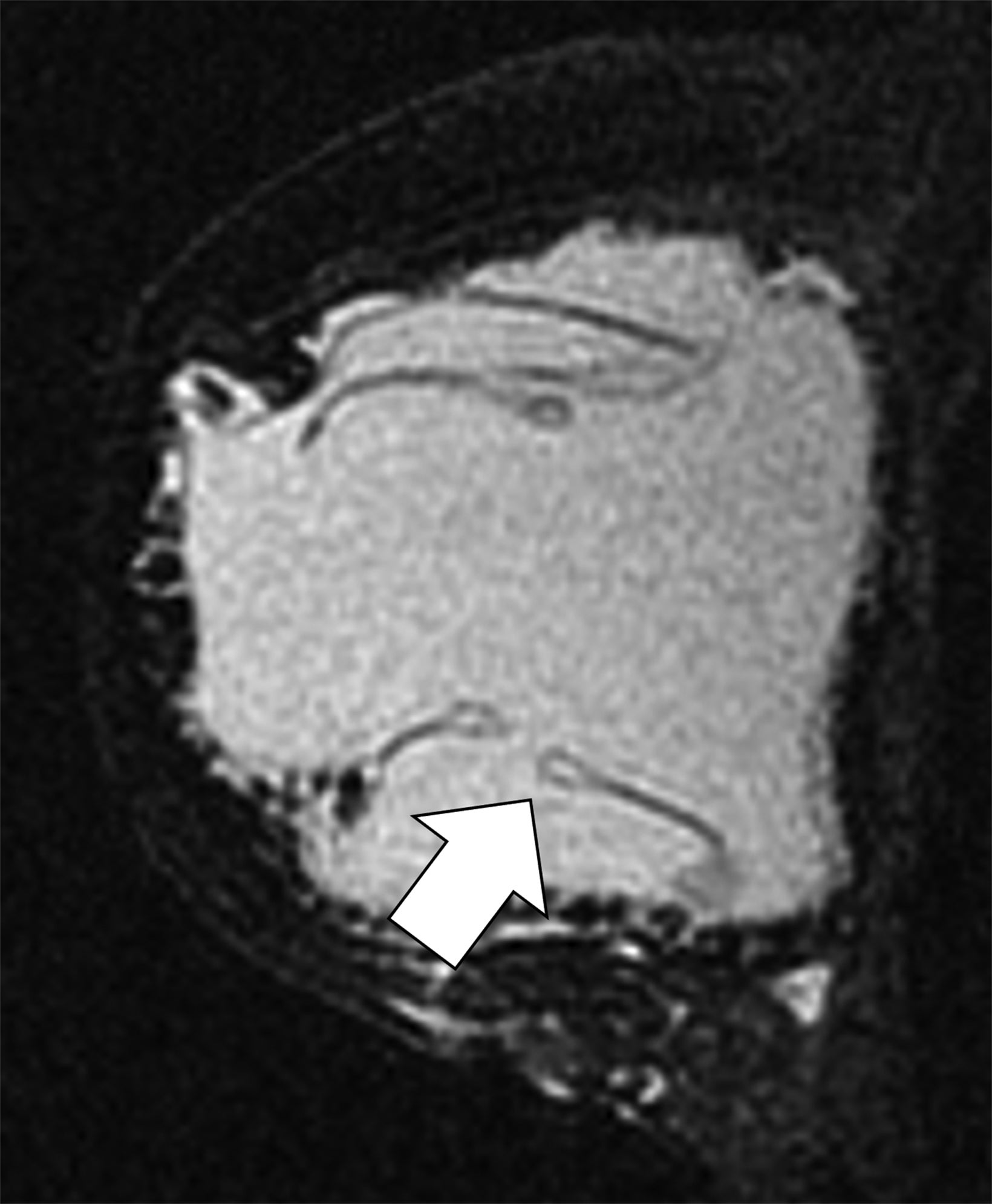
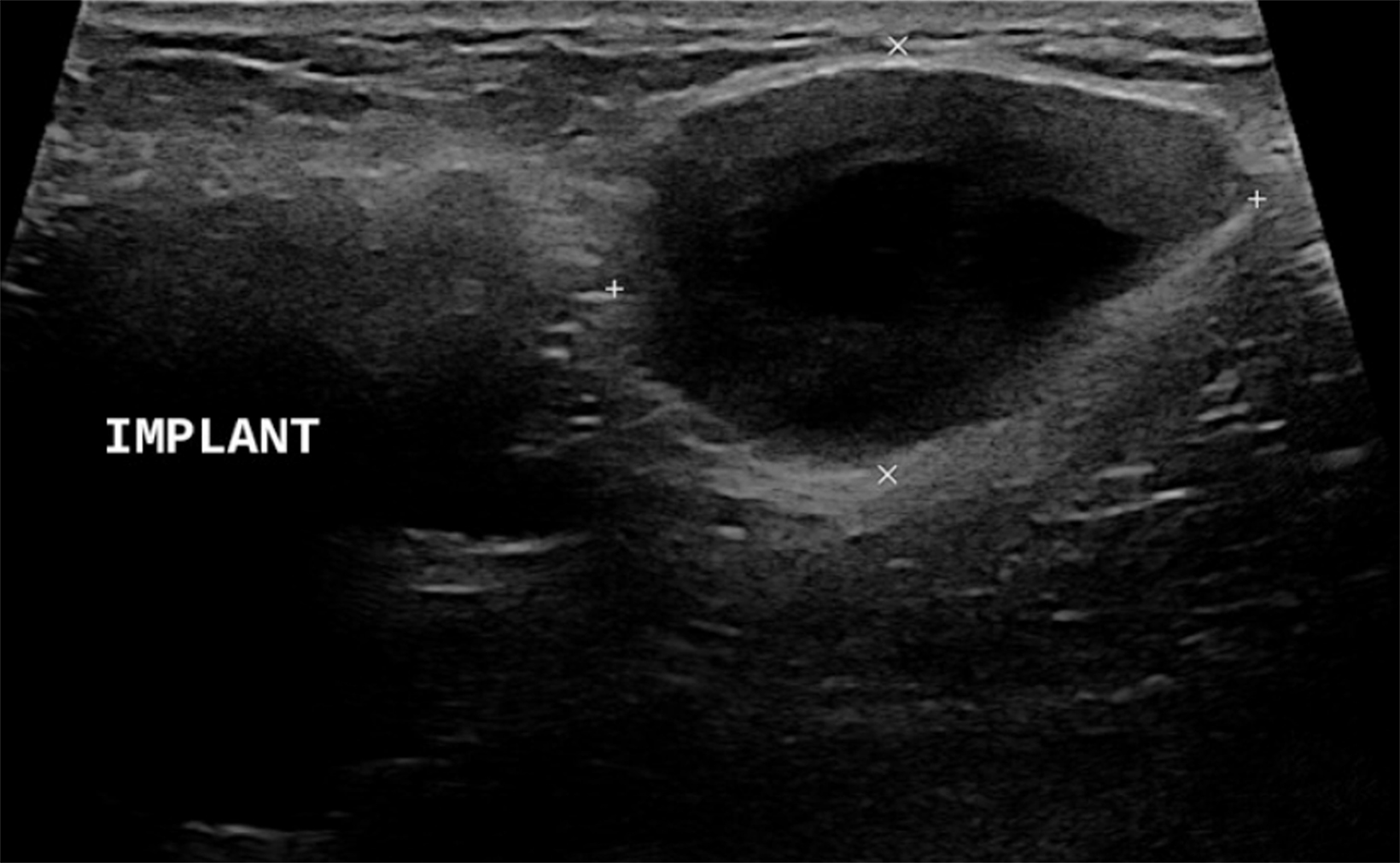
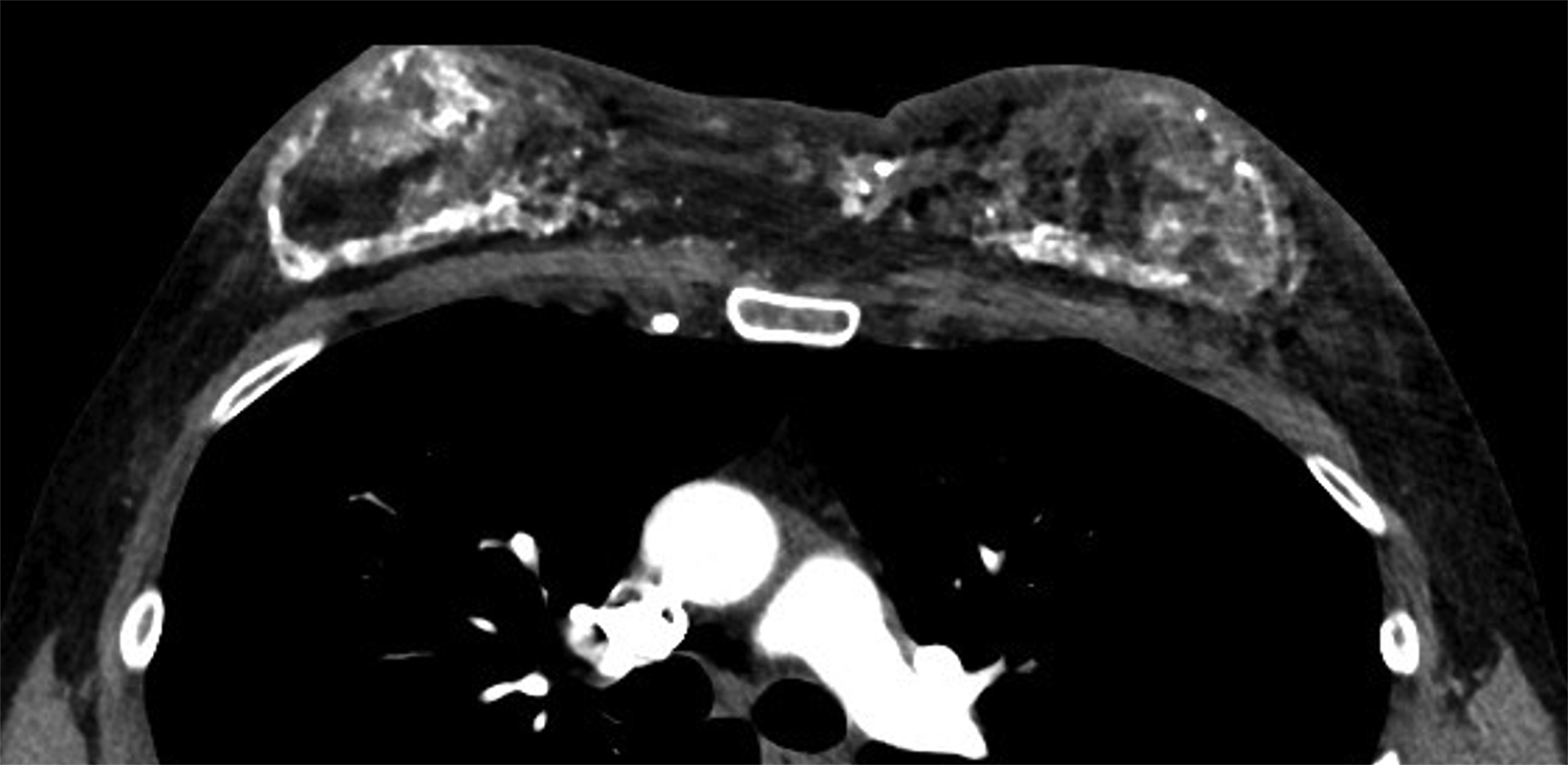
Surgical breast augmentation supplements GAH therapy. About 60% of transgender females seek saline or silicone implants. Non-FDA-approved interventions, including particle injections of free silicone, mineral oil, liquid paraffin, and petroleum jelly,9 can cause breast lumps, infection, and pain. On imaging, these entities appear as multifocal regions of fibrosis and granulomas, resulting in markedly limited evaluation of the breast parenchyma on both mammography and ultrasound. Magnetic resonance imaging (MRI) can be a valuable technique in detecting breast cancer in patients with silicone-granulomas, given the significant limitations of mammography and ultrasound (Figures 4-7).12 Free silicone granulomas do not demonstrate contrast enhancement, which is a critical differentiator from breast cancer.10
Breast Cancer Risk
The risks of long-term GAH therapy and its role in breast cancer development remain poorly understood secondary to a lack of longitudinal population-based studies.13 Transgender females are considered an at-risk population for increased breast cancer morbidity and mortality due to social factors such as a lack of screening awareness by both patients and physicians.
The biological risk of breast cancer development in this population can be considered not only within the context of cisgender males with elevated estrogen levels, owing to factors such as obesity and liver disease, but also within the context of postmenopausal cisgender women on hormone replacement therapy (HRT).14,15 Postmenopausal cisgender women have a greater relative breast cancer risk after four to five years of HRT, implying an increased risk in transgender females on long-term GAH therapy.11, 13,16 Anti-androgen therapy in transgender females may also contribute to cancer risk, as testosterone is a breast tissue apoptotic agent.17
Brown, et al, aimed to examine breast cancer incidence and exposure to GAH therapy in the largest North American sample of transgender female patients to date. Of the 5135 subjects, 3566 identified as transgender females. Breast cancer was detected in three of these subjects, with an average age at diagnosis of 63.8 years. None of these subjects received GAH therapy and all presented at an advanced stage. The researchers concluded that breast cancer incidence in transgender females is comparable to that of cisgender males.18,19
Consensus standard of care guidelines published by the World Professional Organization for Transgender Health categorize the risk level of breast cancer resulting from feminizing hormones as “no increased risk or inconclusive,” meaning that while GAH may increase the risk, the evidence is so minimal that a conclusion cannot be reached.20
Most breast cancers in transgender females are intraductal carcinomas, which demonstrate estrogen receptor (ER) positivity and present more than a decade earlier when compared to cisgender males. The most common presentation is a palpable breast mass.11, 13 De Blok, et al, demon- strated a predilection of ductal origin and estrogen and progestogen receptor positivity in transgender females.15
Screening Recommendations
There is no longitudinal data on screening benefits to transgender females, although they may find mammography gender affirming. The Society for Breast Imaging recommends screening mammography beginning at age 40 in transgender females; these women experience poorer outcomes due to diminished access to and discrimination in healthcare settings.11
The 2021 ACR Appropriateness Criteria® outlines screening recommendations for this population based on risk assessment and stratification. A transgender female with average risk and without a history of current feminizing GAH therapy use is categorized as “usually not appropriate” for screening. A transgender female older than 25 years with a “higher-than-average risk” and no hormone use “may be appropriate” for a screening mammogram and digital breast tomosynthesis (DBT).21 A transgender female with a BRCA mutation has a 6.8% baseline risk of developing breast cancer, which could potentially be increased by feminizing GAH therapy.22
The guidelines issued by the University of California-San Francisco Center for Transgender Care account for the length of GAH therapy and recommend that screening mammography be performed every two years, once the age of 50 and five to 10 years of feminizing hormone use criteria have been met.1
Considerations in Transgender Males
Gender-affirming Therapy
Like their female counterparts, transgender males may choose a variety of gender-affirming medical or surgical interventions to align their external physical appearance with their male identity. Testosterone GAH therapy to promote masculinization results in compositional changes to the breast tissue; these include a decrease in glandular tissue, an increase in fibrous connective tissue, and a relative decrease in adipose tissue relative to cisgender post-menopausal women (Figure 8).23,24 Bilateral mastectomy, also referred to as top surgery, is a commonly performed surgery. Depending on surgical technique, top surgery performed with chest contouring and nipple grafting can leave residual breast tissue in-situ (Figure 9).25
Breast Cancer Risk
There is a paucity of data on breast cancer risk in transgender male patients after top surgery. Cisgender females who have undergone prophylactic bilateral mastectomy demonstrate a markedly decreased risk of developing breast cancer. It is generally agreed that transgender males are also at very low risk of developing breast cancer after undergoing top surgery.26 A small volume of residual tissue frequently remains in patients, and a few cases of breast cancer have been reported in transgender males after bilateral mastectomy.1,27
Gooren, et al, suggest that androgenic GAH therapy can decrease the risk of breast cancer.14,28,29 De Blok, et al, demonstrated a decrease in breast cancer incidence of 0.2% in transgender males.13,29 In 2018, Stone, et al, reviewed 17 cases of breast cancer in transgender men and concluded that individuals undergoing top surgery are at a much lower risk than cisgender females.29
Screening Recommendations
Transgender males may experience gender dysphoria during mammography. Screening recommendations in transgender males who have not had top surgery depend upon individual risk factors. It is recommended that transgender males follow the same guidelines as cisgender women regardless of testosterone hormone therapy.1,21
For those who have undergone top surgery, routine screening is not recommended by current guidelines.1 Top surgery can be subtotal and the risk of cancer in residual breast tissue is based upon individual factors. Some physicians recommend an annual physical chest exam despite a lack of supporting evidence.1
Conclusion
Long-term, longitudinal, population-based epidemiological studies will help to establish breast cancer trends in the transgender population. This is critical in transgender females, as current data suggest GAH treatment may result in an earlier age of diagnosis and a later stage of cancer in relation to cisgender males.
While there is the suggestion that length of feminizing hormone therapy increases the risk of breast cancer in transgender females, further studies are needed for definitive assessment. Patient-centric research is required to better serve this at-risk minority population, and cultural competency is crucial to providing inclusive medical care and reducing healthcare disparities.
References
- Deutsch BD. UCSF transgender care. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people (2016). 2nd ed. https://transcare.ucsf. edu/guidelines. Accessed January 25, 2021.
- Flores AR, Herman JL, Gates GJ, Brown TNT. Williams institute school of law. How many adults identify as transgender in the United States? https://williamsinstitute.law. ucla.edu/publications/trans-adults-united-states/. Accessed January 21, 2021.
- Meerwijk EL, Sevelius JM. Transgender population size in the United States: a meta-regression of population-based probability samples. Am J Public Health. 2017;107(2): e1-e8. doi:10.2105/AJPH.2016.303578.
- Grant JM, Mottet LA, Tanis J, Herman JL, Harrison J, Keisling M. National center for transgender equality and national gay and lesbian task force. Injustice at every turn: a report of the national transgender discrimination survey (2011). www.thetaskforce.org/static_html/downloads/reports/reports/ntds_full.pdf. Accessed January 21, 2021.
- Mayer KH, Bradford JB, Makadon HJ, Stall R, Goldhammer H, Landers S. Sexual and gender minority health: what we know and what needs to be done. Am J Public Health. 2008;98(6):989-995. doi:10.2105/AJPH.2007.127811.
- Luehmann N, Ascha M, Chwa E, et al. A single-center study of adherence to breast cancer screening mammography guidelines by transgender and non-binary patients. Ann Surg Oncol. 2021. doi:10.1245/s10434-021-10932-z.
- Huang SY, Zhang M, David M. Radiology’s engagement with transgender breast imaging: review of radiology practice websites and publications. J Breast Imaging. 2020;2(2):147-151. doi:10.1093/jbi/wbz081.
- Taylor ET, Bryson MK. Cancer’s margins: trans* and gender nonconforming people’s access to knowledge, experiences of cancer health, and decision-making. LGBT Health. 2016;3(1):79-89. doi:10.1089/lgbt.2015.0096.
- onnenblick EB, Shah AD, Goldstein Z, Reisman T. Breast imaging of transgender individuals: a review. Curr Radiol Rep. 2018;6(1):1. doi:10.1007/ s40134-018-0260-1.
- Parikh U, Mausner E, Chhor CM, Gao Y, Karrington I, Heller SL. Breast imaging in transgender patients: what the radiologist should know. Radiographics. 2020;40(1):13-27. doi:10.1148/rg.2020190044.
- Lienhoop T, Green L. Breast imaging in transgender women: a review. Clin Imaging. 2021; 80:283-289. doi: 10.1016/j.clinimag.2021.07.031.
- Helbich TH, Wunderbaldinger P, Plenk H, Deutinger M, Breitenseher M, Mostbeck GH. The value of MRI in silicone granuloma of the breast. Eur J Radiol. 1997;24(2):155- 158. doi:10.1016/s0720-048x(96)01027-3.
- Hartley RL, Stone JP, Temple-Oberle C. Breast cancer in transgender patients: A systematic review. Part 1: Male to female. Eur J Sur Onco. 2018;44(10):1455-1462. doi: 10.1016/j.ejso.2018.06.035.
- Irwig MS. Testosterone therapy for transgender men. The Lancet. Diabetes and Endocrinol. 2017;5(4):301-311. doi:10.1016/S2213-8587(16)00036-X.
- Blok CJM de, Wiepjes CM, Nota NM, et al. Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands. The BMJ. 2019; 365: l1652. doi:10.1136/bmj. l1652.
- Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. NEJM. 1995;332(24):1589-1593. doi:10.1056/NEJM199506153322401.
- Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: evidence and methodological considerations. Epidemiol Rev. 2017;39 (1): 93-107. doi:10.1093/epirev/mxw003.
- NIH surveillance, epidemiology, and end results program. Cancer stat facts: female breast cancer. Seer.cancer.gov/statfacts/html/breast.html. Accessed January 21, 2021.
- Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Can Res Treat. 2015;149,191-198. doi:10.1007/s10549-014-3213-2.
- The world professional association for transgender health. Standards of care for the health of transexual, transgender, and gender nonconforming people (2012). 7th volume. https://www.wpath.org/publications/soc. Accessed January 25,2021.
- Brown A, Lourenco AP, Niell BL, et al. Expert panel on breast imaging. ACR Appropriateness Criteria® transgender breast cancer screening. J Am Coll Radiol. 2021;18(11S): S502-S515. doi: 10.1016/j.jacr.2021.09.005.
- Eismann J, Heng YJ, Fleischmann-Rose K, et al. Interdisciplinary management of transgender individuals at risk for breast cancer: case reports and review of the literature. Clinical Breast Cancer. 2019;19(1): e12-e19. doi: 10.1016/j.clbc.2018.11.007.
- Grynberg M, Fanchin R, Dubost G, et al. Histology of genital tract and breast tissue after long-term testosterone administration in a female-to-male transsexual population. Reproductive BioMedicine Online. 2010;20(4):553-558. doi: 10.1016/j.rbmo.2009.12.021.
- Slagter MH, Gooren LJG, Scorilas A, Petraki CD, Diamandis EP. Effects of long-term androgen administration on breast tissue of female-to-male transsexuals. J Histochem Cytochem. 2006;54(8):905-910. doi:10.1369/jhc.6A6928.2006.
- Wilson SC, Morrison SD, Anzai L, et al. Masculinizing top surgery: a systematic review of techniques and outcomes. Ann Plast Surg. 2018;80(6):679-683. doi:10.1097/SAP.0000000000001354.
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in brca1 and brca2 mutation carriers: the prose study group. J Clin Oncol. 2004;22(6):1055-1062. doi:10.1200/JCO.2004.04.188.
- Sonnenblick EB, Shah AD, Goldstein Z, Reisman T. Breast imaging of transgender individuals: a review. Curr Radiol Rep. 2018;6(1):1. doi:10.1007/s40134-018-0260-1.
- Gooren LJ, van Trotsenburg MAA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. The Journal of Sexual Medicine. 2013;10(12):3129-3134. doi:10.1111/jsm.12319.
- ACR Appropriateness Criteria®. Transgender breast cancer screening. https://acsearch.acr.org/list/GetEvidence?TopicId=283&TopicName=Transgender%20Breast%20Can- cer%20Screening#. Accessed January 21,2021
References
Citation
DL G, J S, KM P.Effects of Gender-affirming Medical and Surgical Therapy on Breast Imaging Findings and Breast Cancer Risk. Appl Radiol. 2022; (3):24-32.
April 26, 2022