Cystic neck masses: A pictorial review of unusual presentations and complicating features
Images





Dr. Masters is a Radiology Resident and Dr. Given is an Associate Professor of Radiology, Department of Radiology, University of Kentucky Chandler Medical Center, Lexington, KY.
Portions of this material were presented as a Scientific Exhibit at the American Society of Neuroradiology (ASNR) Annual Meeting, June 2007, Chicago, IL.
Imaging plays a vital role in the evaluation of cystic neck masses. To aid in the correct preoperative diagnosis and to facilitate appropriate medical management, it is important for radiologists to recognize both the common and the unusual manifestations of these lesions. This pictorial review illustrates the typical appearance of cystic neck masses and the more unusual, uncommon presentations and complications.
Thyroglossal duct dyst
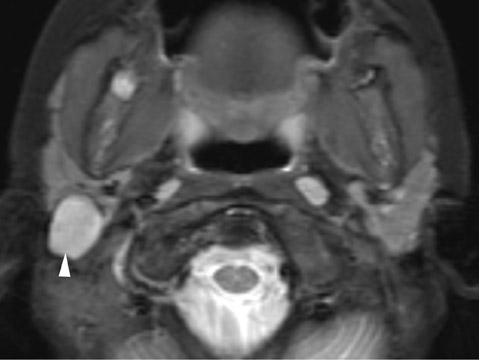
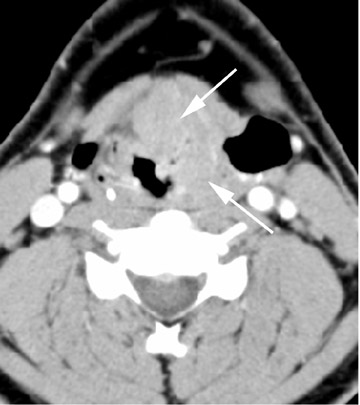
The thyroglossal duct cyst (TDC) is the most common congenital neck mass, compromising 70% of all congenital neck anomalies.1 The thyroid normally descends along the thyroglossal duct, extending from the foramen cecum, through the floor of the mouth, and passes anterior to the hyoid bone, to its final position within the inferior neck. The duct normally involutes during gestation, but if any portion of the duct persists it can give rise to a TDC. The thyroglossal duct is intimately associated with the hyoid bone (Figure 1) and often passes directly through it.2 Clinically, TDCs may migrate cephalad with protrusion of the tongue.2
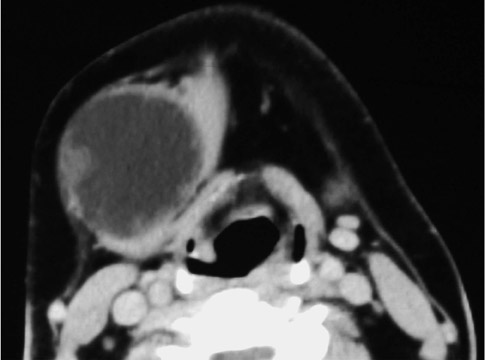
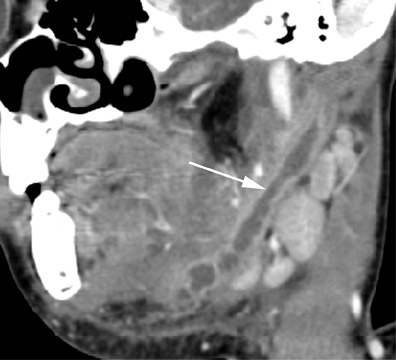
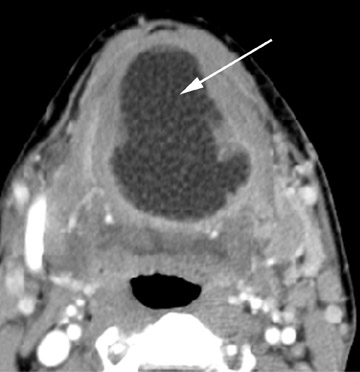
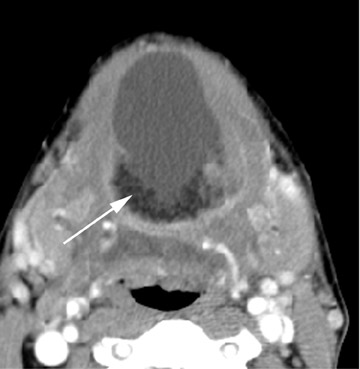
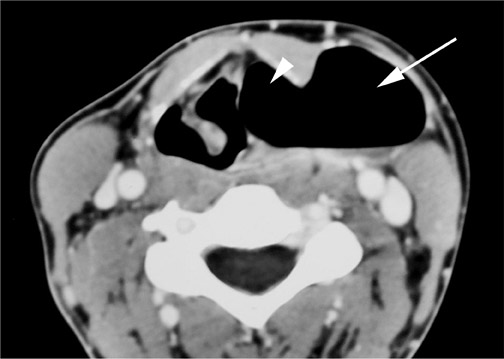
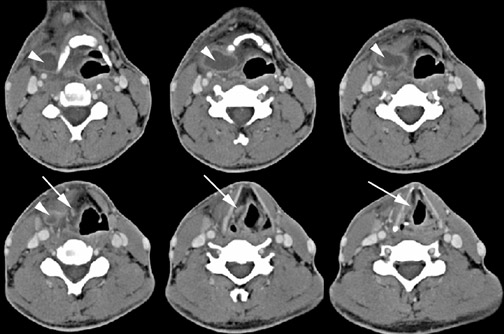
On all imaging modalities, TDCs appear as cystlike masses of the anterior neck, at the level of the hyoid bone or within the strap muscles (Figures 1 through 3). Approximately 75% percent of all TDCs are located in the midline, with 25% within 2 cm of midline.3 With imaging, TDCs generally appear as smooth, well-circumscribed lesions containing simple fluid. Thyroglossal duct cysts may exhibit peripheral enhancement following intravenous contrast administration. With recurrent inflammation, the cysts may become more heterogeneous in appearance and develop internal septations (Figure 2).
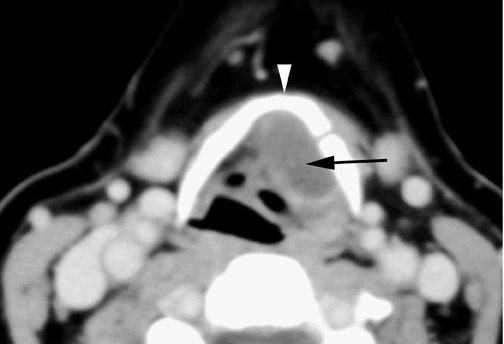
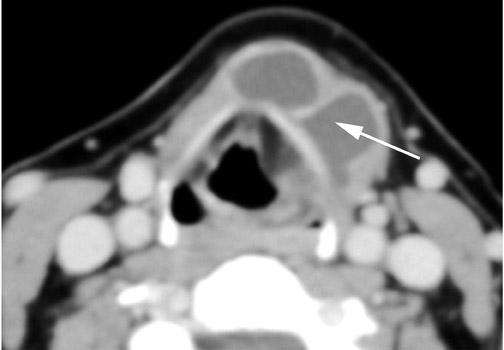
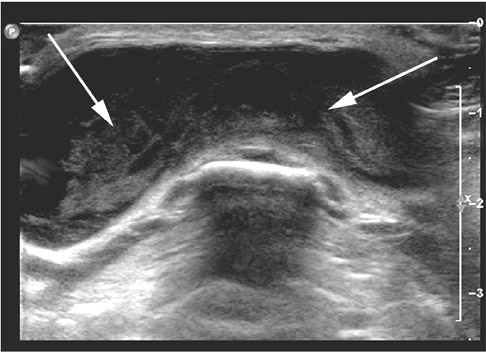
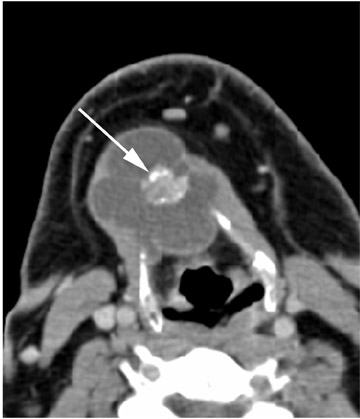
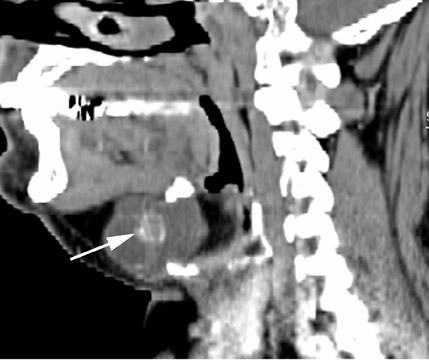
Roughly 1% of TDCs are associated with thyroid carcinoma, most commonly of the papillary subtype.1,3,4 When there are solid soft tissue elements associated with a TDC, often a nodular focus of solid tissue within the dominant cyst (Figure 3), carcinomas arising within TDCs may be suspected.4,5 Carcinomas may also be seen as purely solid masses along the course of the thyroglossal duct. Calcification with the cyst is thought to be a specific finding of carcinoma within a TDC4,5 (Figure 3). In contrast to their traditional counterparts in the thyroid gland, carcinomas arising within TDCs rarely metastasize to cervical lymph nodes.1,4 However, the presence of carcinoma in a TDC may indicate the need for an additional thyroidectomy,6 as up to 14% of patients will have a coincident microscopic focus of papillary carcinoma within the thyroid gland.7
Branchial cleft cysts
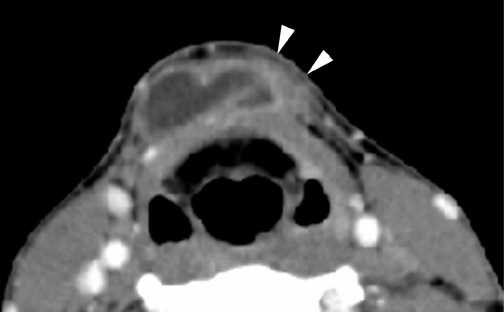
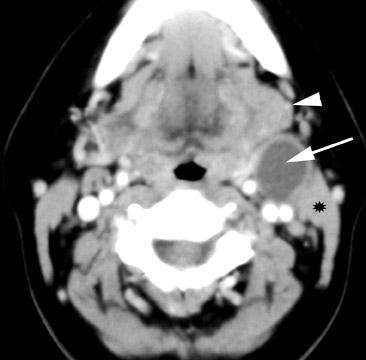
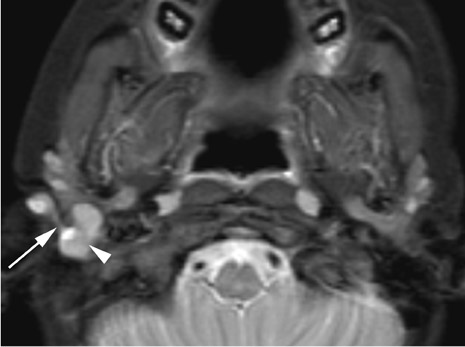
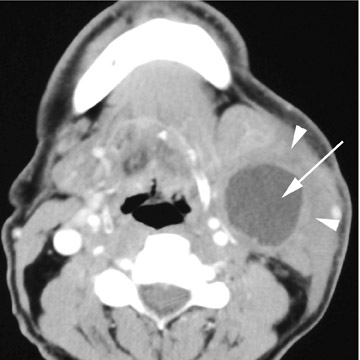
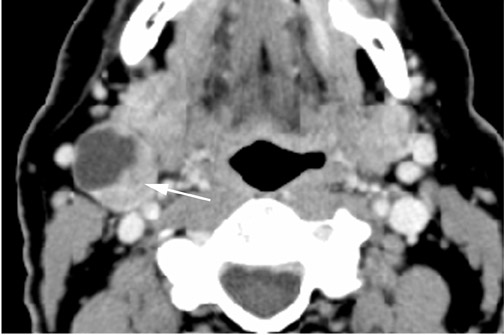
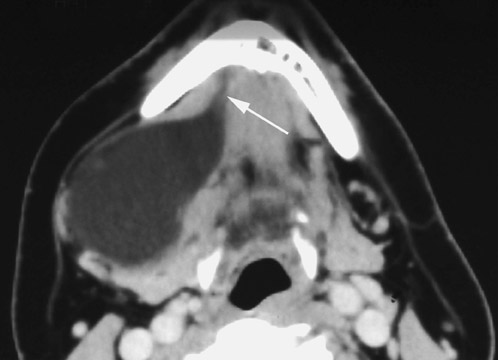
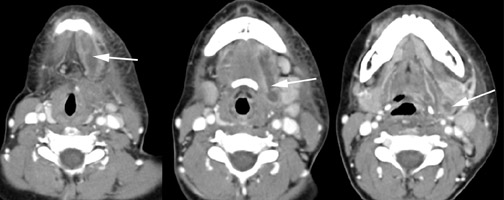
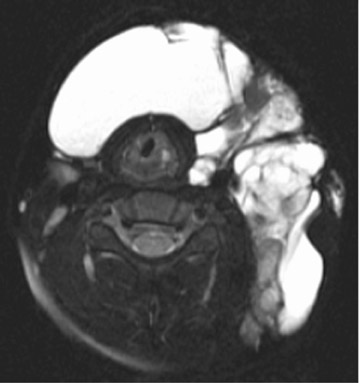
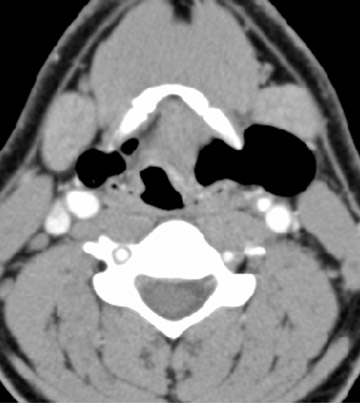
Branchial cleft cysts (BCC) arise from incomplete obliteration of any branchial tract, resulting in either a cyst (75%) or sinus tract (25%).8 Second branchial cleft anomalies comprise 95% of all branchial cleft lesions, classically presenting as cystic masses at the anterolateral border of the sternocleidomastoid muscle, lateral to the carotid space and at the posterior margin of the submandibular gland (Figure 4). Less common branchial cleft anomalies include a first BCC, or a parotid lymphoepithelial cyst, arising from a tract extending from the external auditory canal through the parotid to the submandibular triangle (Figure 5). Third and fourth BCCs are exceedingly rare. Third BCCs are posterior to the common or internal carotid artery and the sternocleidomastoid muscle, between the hypoglossal nerve below and the glossopharyngeal nerve above.9 Fourth BCCs are generally sinus tracts or fistulas and arise from the pyriform sinus, pierce the thyrohyoid membrane, and descend along the tracheoesophageal groove. Branchial cleft cysts may become infected and exhibit thickening and enhancementof the cyst wall (Figure 6), mimicking a suppurative lymph node.
The existence of a primary carcinoma (Figure 7) arising within a BCC remains controversial. Most suspected cases are likely metastatic lesions from occult primaries. Imaging is generally insufficient to differentiate cystic nodal metastases from primary branchiogenic carcinomas, with both exhibiting soft tissue components associated with a cystic lesion. Khafif10 proposed that to make a diagnosis of a primary carcinoma within a BCC there must be no identifiable primary carcinoma elsewhere, and that the cystic lesion must show transition from normal epithelium to invasive carcinoma (transitional zone) on cytopathologic analysis.
Ranulas
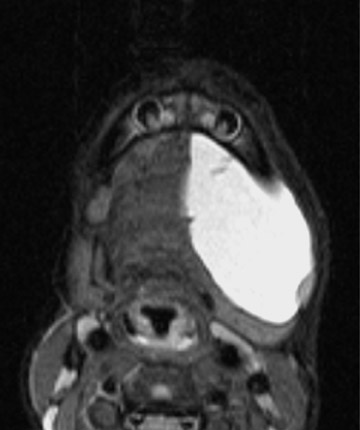
Ranulas represent cystic lesions of the floor of mouth, usually occurring secondary to obstruction of the sublingual duct. Ranulas are classified as either “simple” and confined to the sublingual space, or as “plunging” with extension through the floor of mouth and below the mylohyoid muscle. The plunging variety presents as a cystic mass centered within the submandibular space (Figure 8).
Rarely, ranulas can dissect across the midline between the mylohyoid and geniohyoid muscles to present as a bilateral mass (Figure 9). Ranulas may become infected and demonstrate a thick, irregular rim of enhancement with surrounding inflammatory change (Figure 10). Because of the lack of a fascial boundary between the sublingual/submandibular and the parapharyngeal spaces, plunging ranulas may uncommonly extend into the parapharyngeal space11 (Figure 10).
Cystic hygroma
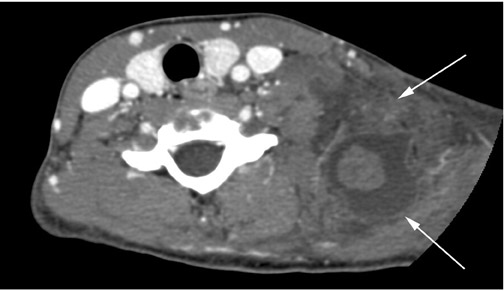
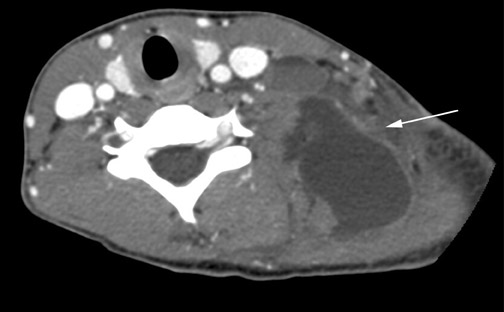
Cystic hygromas represent the most common form of a lymphangioma. These lesions are thought to arise from an early sequestration of embryonic lymphatic channels,12 most commonly occurring along the developing jugular chain. Cystic hygromas typically appear as a multilocular cystic mass with septations of variable thickness, usually centered in the posterior triangle or submandibular space. These lesions are characteristically infiltrative in nature and do not respect fascial planes (Figure 11). Fluid levels may be present when the lesions are complicated by hemorrhage, and large lesions may cross the midline (Figure 11). Cystic hygromas may become infected and increase in size (Figure 12).
Dermoid and epidermoid cysts
Dermoid and epidermoid cysts may occur anywhere in the body, with 7% presenting as head and neck lesions, most commonly lateral to the eyebrow.9 Approximately 11% of the head and neck lesions are located within the floor of the mouth.9 The floor-of-the-mouth lesions typically present as thin-walled, unilocular masses located in the submandibular or sublingual space. There may be coalescence of fat into small nodules within the cystic lesion, giving a “sac-of-marbles” appearance (Figure 13). The rim of the cyst may show contrast enhancement. It has been noted that 5% of lesions may undergo malignant degeneration into squamous cell carcinomas.13
Laryngocele
The laryngeal ventricle is a slit like cavity, with the orifice located between the true and false cords. A laryngocele is dilatation of the laryngeal saccule, a small pouch arising from the roof of the ventricle.14 Laryngoceles confined to the larynx are known as internal laryngoceles. Those that extend through the thyrohyoid membrane, but with dilation of only the extralaryngeal component are termed external . Mixed laryngoceles have dilatation of the saccule on both sides of the thyrohyoid membrane (Figure 14).
Laryngoceles generally present as round or oval lesions within the superior paralaryngeal space (Figure 14) and may have an extralaryngeal component (external and mixed). The laryngocele may be completely air-filled, fluid-filled, or have an air-fluid level (Figure 14). Fifteen percent of laryngoceles are associated with carcinoma,15 with tumor occluding the orifice of the laryngeal ventricle (Figure 15). The presence of soft tissue within a laryngocele suggests an underlying neoplasm. In as many as 8% to 10% of cases,16 a laryngocele may become infected, called a laryngopyocele (Figure 16).
Thymic cyst
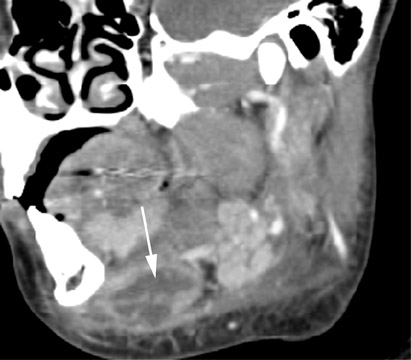
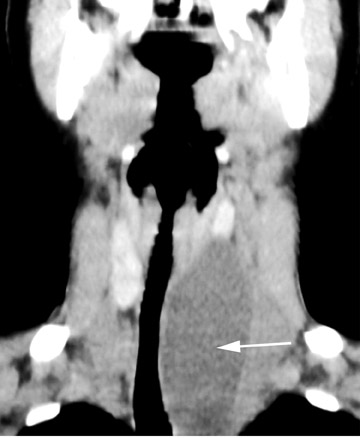
Thymic cysts have a disputed etiology, but most favor the theory that these lesions arise from persistence of the thymopharyngeal duct,17 adjacent to the carotid sheath from the angle of the mandible to the thoracic inlet.18 The cysts arise from the third and fourth branchial clefts,and, therefore, thymic cysts may have a similar appearance to third and fourth branchial cleft cysts, being differentiated only by the presence of thymic tissue within thymic cysts. The cysts usually present as a unilocular cystic mass extending inferiorly within the neck, paralleling the sternocleidomastoid muscle (Figure 17). There are no reports of myasthenia gravis or neoplasia associated with thymic cysts.9
Conclusion
Patients with palpable neck masses are frequently sent for evaluation with cross-section imaging both for diagnosis and for planning biopsy or surgical resection. It is important for radiologists to not only recognize the common appearance of cystic neck masses, but to appreciate the more unusual manifestations of such masses and potential complications that may alter therapy or surgical management.
REFERENCES
- Allard RH. The thyroglossal cyst. Head Neck Surg. 1982;5:134-146.
- Filston HC. Common lumps and bumps of the head and neck in infants and children. Pediatr Ann. 1989;18:180-186.
- Telander RL, Filston HC. Review of head and neck lesions in infancy and childhood. Surg Clin North Am. 1992;72:1429-1447.
- Fernandez JF, Ordonez NG, Schultz PN, et al. Thyroglossal duct carcinoma. Surgery. 1991;110: 928-934; discussion 934-935.
- Glastonbury CM, Davidson HC, Haller JR, Harnsberger HR. The CT and MRI imaging feature of carcinoma arising in thyroglossal duct remnants. AJNR Am J Neuroradiol. 2000;21:770-774.
- Kennedy TL, Whitaker M, Wadih G. Thyroglossal duct carcinoma: A rational approach to management. Laryngoscope . 1998;108(8 Pt 1):1154-1158.
- Wexler MJ. Surgical management of the thyroglossal duct carcinoma: Is an aggressive ap-proach justified? Can J Surg. 1996;39:263-264.
- Deane SA, Telander RL. Surgery for thyroglossal duct and branchial cleft anomalies. Am J Surg. 1978136:348-353.
- Koeller KK, Alamo L, Adair CF, Smirniotopoulos JG. Congenital cystic masses of the neck: Radiologic-pathologic correlation. RadioGraphics. 1999; 19: 121-146; quiz 152-153.Erratum in: Radio Graphics. 1999;19:282.
- Khafif RA, Prichep R, Minkowitz S. Primary branchiogenic carcinoma. Head Neck. 1989;11: 153-163.
- Kurabayashi T, Ida M, Yasumoto M, et al. MRI of rannulas. Neuroradiology. 2000;42:917-922.
- Smith D. Recognizable Patterns of Human Malformation: Genetic, Embryologic, and Clinical Aspects , 3rd ed. Philadelphia, PA: WB Saunders; 1982:472-473.
- Som P. Cystic lesions of the neck. Postgrad Radiol. 1987;7:211-236.
- Delahunty JE, Cherry J. The laryngeal saccule. J Laryngol Otol. 1969;83:803-815.
- Canalis RF, Maxwell DS, Hemenway WG. Laryngocele-An updated review. J Otolaryngol. 1977;6:191-199.
- Stell PM, Maran AG. Laryngocoele. J Laryngol Otol. 1975;89:915-924.
- Zarbo RJ, McClatchey KD, Areen RG, Baker SB. Thymopharyngeal duct cyst: A form of cervical thymus. Ann Otol Rhinol Laryngol. 1983;92:284-289.
- Mikal S. Cervical thymic cyst. Case report and review of the literature. Arch Surg. 1974;109:558-562.