Cavities in the Lung in Oncology Patients: Imaging Overview and Differential Diagnoses
Images
Dr. Gill is an Associate Radiologist, Department of Radiology, Brigham and Women’s Hospital, Boston, MA, and an Instructor of Radiology, Harvard Medical School, Boston, MA; Dr. Matsusoka is a Visiting Assistant Professor, Department of Radiology, St. Marianna University School of Medicine,Kawasaki, Kanagawa, Japan; and Dr. Hatabu is Clinical Director, MRI Program, and Medical Director, Center for Pulmonary Functional Imaging,Department of Radiology, Brigham and Women's Hospital, Boston, MA, and Associate Professor of Radiology, Harvard Medical School, Boston, MA.
A cavity has been defined as “a gas-filled space within a pulmonary consolidation, a mass, or a nodule, produced by the expulsion of [the] necrotic part of the lesion via the bronchial tree.”1 In oncology patients, cavitary lesions caused by various etiologies are seen, and an accurate diagnosis often can be challenging because the nonmalignant cavitary lesions often mimic malignant cavitary lesions. However, the radiological findings of cavitary lesions can be useful in differentiating a cavitary malignant lesion from any other nonmalignant cavitary lesion. This review focuses on the cavitary lesions that are encountered in oncology patients, including primary bronchogenic carcinoma, pulmonary metastasis, infections and other miscellaneous etiologies causing cavitation in oncology patients. The article will highlight radiological findings that aid in diagnosis.
Primary lung cancer
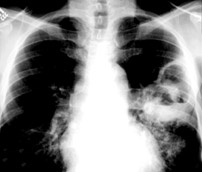
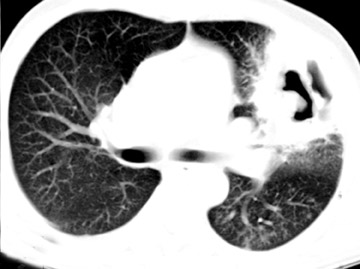
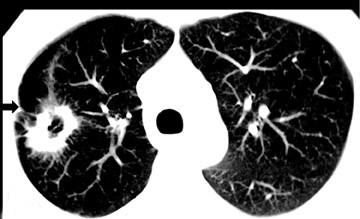
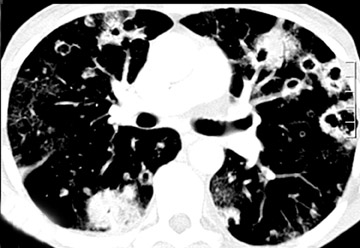
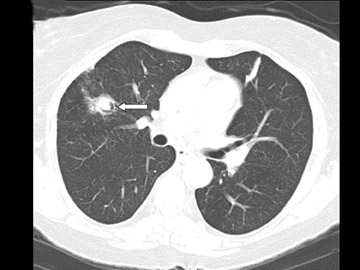
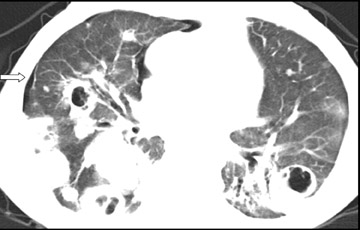
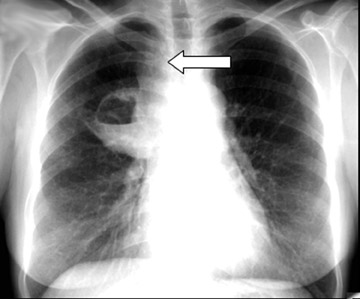
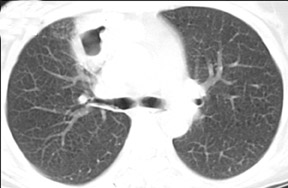
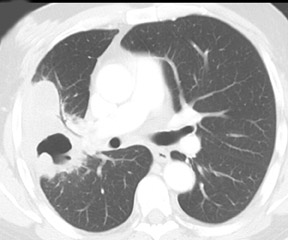
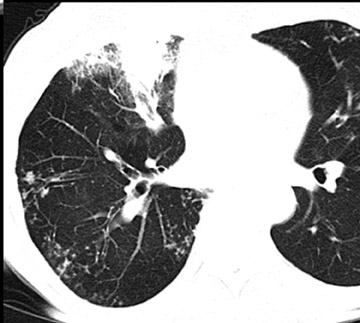
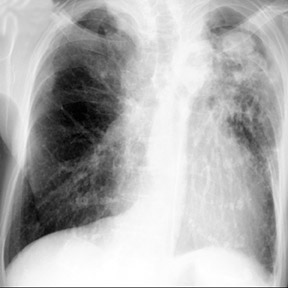
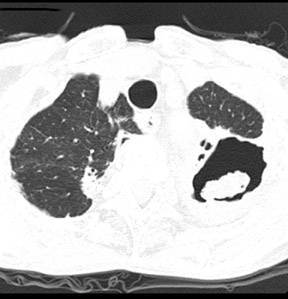
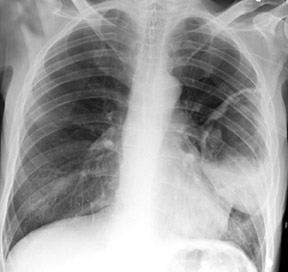
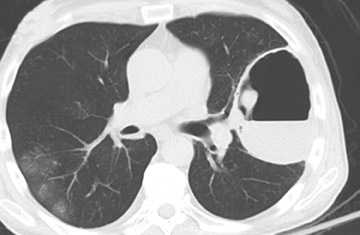
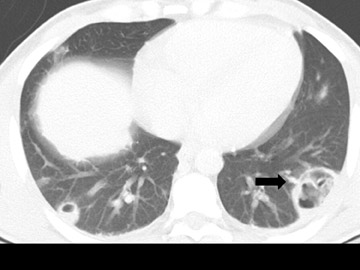
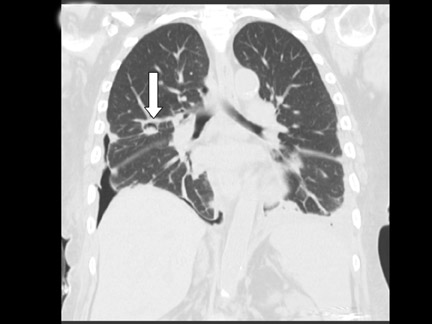
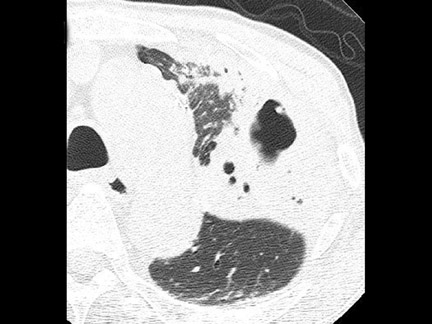
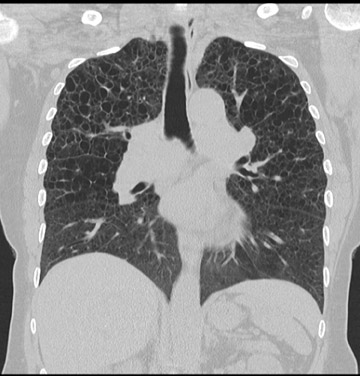
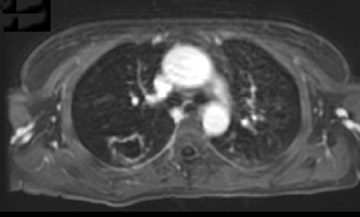
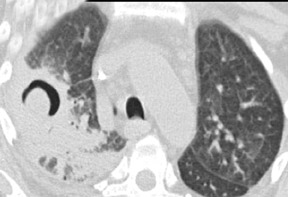
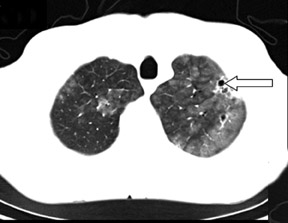
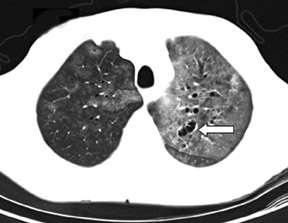
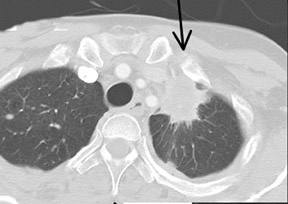
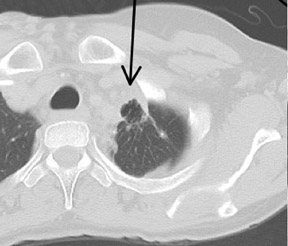
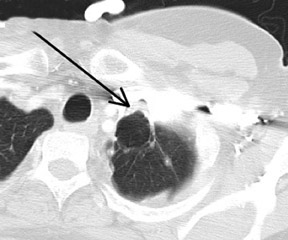
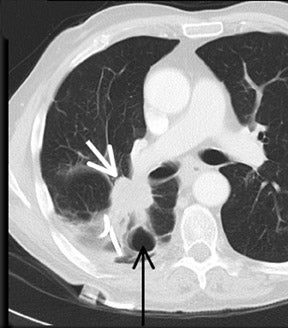
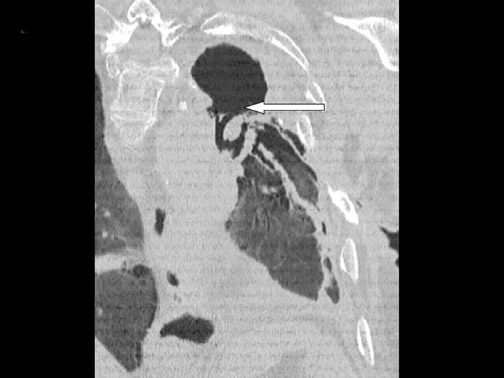
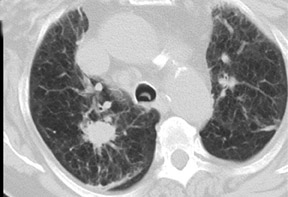
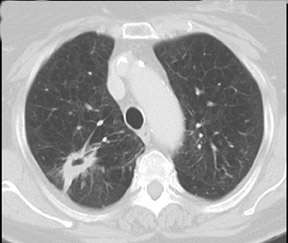
Cavitation in primary lung cancer is not rare. Cavitation detected on plain chest radiographs has been reported in 2% to 16 % of primary lung cancers,2-7 and it is detected with computed tomography (CT) in 22% of primary lung cancers.8 squamous-cell carcinoma is the most common histological type of lung cancer to cavitate (82% of cavitary primary lung cancer, Figure 1), followed by adenocarcinoma (Figure 2) and large cell carcinoma.4,9 Multiple cavitary lesions in primary lung cancer are rare, however, multifocal bronchoalveolar cell carcinoma can occasionally have multiple cavitary lesions (Figure 3).10,11 Small cell carcinoma is never known to cavitate.12
Cavitation can be emphasized by a check-valve mechanism developed as a consequence of tumor infiltration into the bronchial tree.13 Although the mechanism of cavity formation is often difficult to ascertain, cavitation in lung cancer most often results from rapid tumor growth that exceeds the blood supply with resultant central necrosis. A recent study showed that 81% of tumors with cavitation were associated with over-expression of epidermal growth factor receptor (EGFR), and the high level of EGFR expression in these tumors might be associated with rapid growth, central necrosis and formation of cavitation.8 The presence of cavitation in primary lung cancer has been associated with a worse prognosis.14
In general, the radiological features of cavitation that suggest malignancy include wall thickness, and spiculated or irregular inner and outer margins. Previous studies with plain chest radiography showed that the measurement of the cavity wall thickness at its thickest section was most useful in predicting whether the cavity was malignant or benign.15,16 In these studies, 30 of 32 cavities (94%) with a maximum wall thickness of ≤4 mm were caused by a benign process. Cavities with a maximum wall thickness of 5 mm to 15 mm were mixed, with 33 of 55 (60%) benign and 22 of 55 (40%) malignant. Similarly, 35 of 39 cavities (90%) with a maximum wall thickness >15 mm were usually malignant.
Another recent study reported the result of differentiation of malignant from benign cavitary nodules using CT findings.17 In this study, the notch was found in 29% of benign cavitary nodules and in 54% of malignant cavitary nodules (p< 0.01). An irregular internal wall was found in 26% of benign nodules and in 49% of malignant nodules (p< 0.01). In addition, a linear margin, satellite nodules, bronchial wall thickening, consolidation, and ground-glass attenuation were significantly more frequent in benign cavitary lesions than in malignant ones. Although these findings might be helpful in differentiating cavitary nodules to some degree, the CT findings of benign and malignant cavitary nodules can overlap.
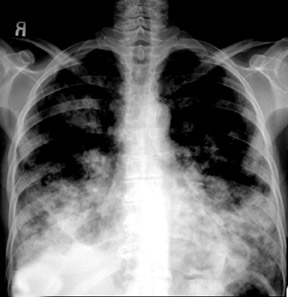
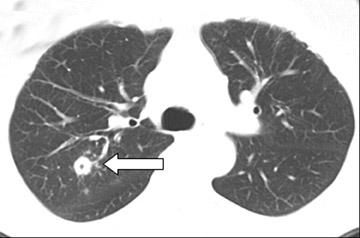
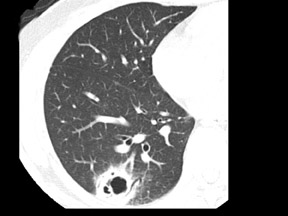
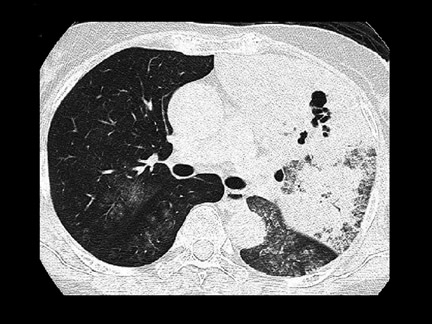
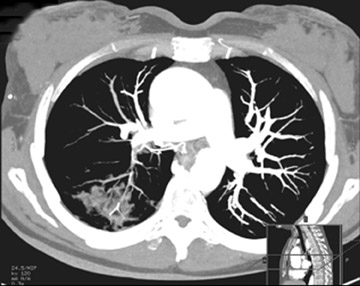
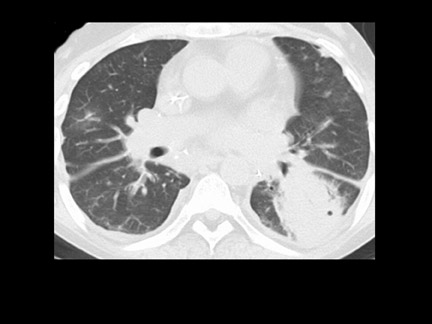
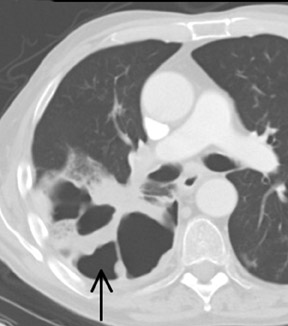
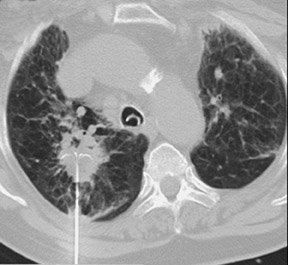
Unusual findings of primary cavitary lung cancer include thin-wall cavitation.9 Although the “air-crescent sign” (a collection of air in a crescent-like shape that separates the wall of a cavity from an inner mass) is most often associated with an inflammatory process (e.g., mycetoma, a hydatid cyst, or pulmonary tuberculosis18), lung cancer can also have an air-crescent sign in rare cases.19-20 In such cases, positron emission tomography (PET) images can help in the differential diagnosis.19 Contrarily, mycetoma also can arise from a cavitary lesion within lung cancer.21 In fact, mycobacterial or fungal pathogens can also coexist in malignant cavities (Figure 4).22-24
Pulmonary metastasis
Metastatic lung lesions also can cavitate, but this occurs less frequently than in primary lung cancers. The frequency of cavitation in metastatic tumor detected by plain radiograph is 4%.2 Cavitary lung metastasis can occur in any histological type, however, squamous-cell carcinoma is the most common cause of cavitating metastases, comprising 69% of these instances.25 The mechanism of the cavitation has not been completely clarified. In metastatic tumors from squamous carcinoma, cavitation might result from cornification of the squamous epithelium in the center of the lesion with subsequent liquefaction and evacuation into airway.26 This process could explain the tendency of head-and-neck squamous cancer metastases to produce cavities more often than other squamous-cell carcinoma sites.
Morphologically, cavitating metastases have a thick and irregular wall, but thin-walled cavities can be observed with metastases from sarcomas and adenocarcinomas.25 Metastatic sarcomas with cavitation also can be complicated by a pneumothorax (Figure 5). The wall thickness of cavitary metastasis may remain constant as the diameter of the cavity increases.2 Cavitation occurs more often in the upper lobe than in the lower lobe.27 Generally, cavitating pulmonary metastases present as multiple lesions, but can present as a single cavitary lesion.26 In such cases, accurate diagnosis with only radiological findings is extremely difficult and the clinical history or histopathological analysis post biopsy is needed.
Other malignant diseases
Cavitary lesions in patients with lymphoma are infrequent, however, pulmonary lymphoma can cavitate (Figure 6).28 These cavities are usually multiple with thick walls and have an upper-lobe predominance.29, 30 However, in lymphoma patients with human immunodeficiency virus (HIV) infection, cavitation is not rare, and ≤25% of patients can have a cavitary lesion detected on CT images.31
Nonmalignant cavitary diseases
Tuberculous, nontuberculous, bacterial and fungal infections can present with cavity formation and ascertaining the most likely diagnosis is vital in the management of the oncology patients, especially the post bone-marrow transplant population.
Mycobacterium tuberculosis infection
Cavitation is common in post primary tuberculosis and is usually located in the apical and posterior segments of the upper lobes and superior segments of the lower lobes.32,33 The prevalence of cavities on plain chest radiographs varies in 30% to 50% of patients. Cavities can vary widely in size and have been reported to have both thick and thin walls.34-36 Multiple cavities are often present and frequently occur in areas of consolidation.34,37 The presence of cavitation is associated with a greater degree of infectiousness, likely due to higher organism burden.38 The number and maximum size of cavities can correlate with the numbers of acid-fast bacilli (AFB) in sputum.39 Although the morphological findings of cavitation in patients with post primary tuberculosis are hardly distinguishable from malignant cavitary lesions (Figure 7), the presence of adjacent tree-in-bud lesions,40 or satellite nodules may help in differentiating tuberculosis from malignancy. The efficacy of dynamic CT and magnetic resonance imaging (MRI) has been reported to differentiate between malignant tumor and tuberculoma,41.42 but it has not been clarified whether the presence of cavitation affects that efficacy or not.
Factors that depend on the host play an important role in the prevalence of cavitation of tuberculosis. In patients with acquired immunodeficiency syndrome, cavitation is less frequent,43,44 whereas cavitation is highly prevalent among diabetic patients with tuberculosis,45 and multiple small, irregular cavities also have been reported on CT scans.46
Nontuberculous mycobacterial infection
Nontuberculous mycobacteria including Mycobacterium kansasii and Mycobacterium avium-intracellularecomplex can cause pulmonary infections that are associated with cavities. As for Mycobacterium avium-intracellulare complex, 65% of patients have cavitation (Figure 8), and the presence of cavity on CT is associated with positive sputum culture.47 The cavities are relatively small and thin walled.48,49 Sometimes it is difficult to differentiate from cavitary metastasis. However, other typical CT findings, including nodules with associated bronchiectasis particularly in the lingula and right middle lobe, may help in the diagnosis.47,50
In patients with Mycobacterium kansasii, the frequency of cavitation is high (87% to 96%) and cavitation is visible even on plain radiographs. Cavitary lesions may be single or multiple with thin walls, predominantly in the upper lobes.51,52
Fungal infections
The radiological presentations of pulmonary fungal infections vary depending on the patient’s immune conditions. In the immunocompetent patient, fungal infections are uncommon, however, cavitation in fungal infection is not rare and can mimic malignant cavity. Thus, occasionally differentiating a fungal cavity from malignant cavity is difficult.
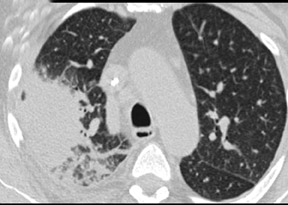
An aspergilloma represents growth of aspergillus within a pre-existing lung cavity. The typical radiographic finding is a rounded soft tissue within a previously existing cavitary lesion such as tuberculosis cavity (Figure 9). The appearance of the soft tissue can be similar to malignant lesion. It has been reported that the enhancement of the intramural soft tissue on CT implies malignancy, while the findings of adjacent bronchiectasis, a dependent location and positional mobility suggest aspergilloma.53
The radiological findings of cryptococcosis also depend on the immune status of the patient. The most common findings in immunocompetent patients are focal infiltration and nodules. Cavitation can be detected in 14% to 42% within consolidation and nodules in immunocompetent patients (Figure 10). There are no specific findings that can differentiate cavitary cryptococcosis from malignant cavitary lesion. On the other hand, cavitation is significantly less common in severely immunocompromised patients, particularly in HIV patients, than in immunocompetent ones.54-56 However, in mild to moderately immunocompromised patients such as diabetes, liver cirrhosis, and corticosteroid therapy, cavitation is observed more frequently (62.5% of patients).54
Cavities with thick or thin walls are seen in patients with pulmonary blastomycosis, histoplasmosis, coccidioidomycosis, and mucormycosis.57-60 These cavitary lesions can be solitary or multiple. Unfortunately, there are no specific findings that can differentiate between these cavitary fungal nodules and malignant cavities.
Bacterial infections
Cavitation can be seen with community-acquired bacterial pneumonia, particularly Klebsiella pneumoniae pneumonia (Figure 11) and Staphylococcus aureus pneumonia. However, most cases of community-acquired bacterial infection present symptomatic inflammatory changes such as productive cough and high-grade fever. In addition, rapid change of ill-defined consolidation on plain chest radiography suggests bacterial pneumonia rather than malignancy.
Pulmonary actinomycosis usually results from aspiration of infected material containing actinomyces.61 Cavitation in actinomycosis is a common finding on CT in 62% to 75%.62,63 Pulmonary actinomycosis is relatively asymptomatic and progresses gradually with nonproductive cough or low-grade fever. In addition, chest-wall invasion, transfissural extension, and hilar or mediastinal lymphadenopathy can be occasionally observed.64 Segmental consolidations that contain low-attenuation areas with peripheral enhancement and adjacent pleural thickening suggest pulmonary actinomycosis.62
Septic embolism usually presents with severe symptoms including high-grade fever and dyspnea, however, asymptomatic cases also have been reported.65 Cavitary nodule located in the lung periphery is frequent and can be detected in 85% of instances on CT.66 The appearance can be confused with cavitary malignant lesion such as multiple pulmonary metastases (Figure 12). Classically, “a feeding vessel sign,” in which a distinct vessel is seen leading to the center of a pulmonary nodule, has been reported as a typical finding of septic emboli.67 however, the feeding vessel sign also can occur in pulmonary metastasis.68 In addition, a recent study showed that most of these vessels coursed around the nodule and some were pulmonary veins.69
Meanwhile, the pneumonic type of bronchoalveolar cell carcinoma shows lobar consolidation, and both bacterial pneumonia and the pneumonic type of bronchoalveolar cell carcinoma can have cavitations. Thus, it is often difficult to differentiate from bacterial pneumonia (Figure 13). In old times, the “angiogram sign,” in which branching pulmonary vessels could be visualized normally within areas of consolidation on contrast-enhanced CT, was reported as a specific sign of the pneumonic type of bronchoalveolar cell carcinoma,70 however, it also can be seen in pneumonia.71 A recent study showed that consolidation on CT may suggest infectious pneumonia rather than the pneumonic type of bronchoalveolar cell carcinoma when bronchial wall thickening, proximal to the lesion, and pleural thickening, associated with the lesion, is evident.72
Noninfectious inflammatory diseases
In rheumatoid patients, nodular lung disease in the form of necrobiotic (“rheumatoid”) nodules can be encountered. Patients with pulmonary necrobiotic nodules are typically men with clinical and radiographic evidence of rheumatoid arthritis, including subcutaneous nodules, high rheumatoid factor, and pulmonary interstitial pneumonia. Usually, pulmonary rheumatoid nodules are multiple, involving the lower lobes of the lungs, and can cavitate (Figure 14). Recently, an association between rheumatoid disease and several types of malignancy has been confirmed,73 and necrobiotic nodules may be indistinguishable radiologically from malignant lesions. Therefore, it has been recommended that all pulmonary nodules in rheumatoid patients be biopsied.74
Wegener’s granulomatosis can present as multiple masses or a single mass with cavitation.75.76 Cavitation of the nodules occurs in approximately 50% of cases. The cavities usually have irregular, thick walls (Figure 15).77 Cavitary nodules represent active inflammatory lesions,78 and with treatment the nodules or cavities may resolve completely or result in a scar.77 There are several reports of Wegener’s granulomatosis being misdiagnosed as primary lung cancer and metastasis.79.80 Radiologically, it may be difficult to differentiate between malignant lesion and Wegener’s granulomatosis. However, in patients with Wegener’s granulomatosis, proteinase 3–anti-neutrophil cytoplasmic antibodies (PR3-ANCA) are positive in >90% of cases.81
Eosinophilic granulomatosis (EG) can incidentally be found with malignancy and has been found to coexist with lymphoma.82 EG can present as nodules and small cavitary lesions generally <10 mm and mediastinal adenopathy in ≤30% of patients; the hallmark of the disease is sparing of costophrenic sulci (Figure 16).
Miscellaneous diseases with cavitation
Pulmonary infarction occurs in nearly one third of patients with pulmonary embolism, and cavitation can be detected in ≤32% of patients with pulmonary infarction on CT scans.83 Cavitation in infarction is located in the lung periphery, and there is evidence of pulmonary embolus, right heart strain and pleuritic chest pain, but no specific radiographic findings related to the cavity.84 Oncology patients are at increased risk for pulmonary emboli,85 however, the frequency of pulmonary infarction and cavitation has not been reported in this group (Figure 17).
Cavitary lesions associated with oncotherapy
Oncology patients who undergo chemotherapy and/or radiotherapy often have a malfunctioning immune system. Under immunosuppression, morphological appearances of pulmonary infectious diseases are different from that in immunocompetent patients.
Invasive pulmonary aspergillosis afflicts severely immunocompromised patients, especially those with hematological malignancies or bone marrow transplant recipients. Cavitation generally emerges later in the course of the disease and is often noted during recovery from neutropenia – it is generally considered a treatment response to antifungal therapy.86.87 The air crescent sign is often considered characteristic of retraction of infected lung in invasive aspergillosis (Figure 18).88 However, this sign can be seen in other conditions such as tuberculosis, Wegener’s granulomatosis and lung cancer. In addition, it has been reported that the presence of a “halo sign,” defined as a nodule surrounded by ground-glass attenuation, is reasonably sensitive and specific for invasive aspergillosis in high-risk patients.89 At pathologic examination, the nodules represent foci of infarction, and the halo of ground-glass attenuation results from alveolar hemorrhage.90 However, this CT appearance has been observed in patients with lung metastases from angiosarcoma, choriocarcinoma and osteosarcoma.91
Pneumocystis jiroveci pneumonia is a common opportunistic infection in HIV-infected patients but can also occur in patients with hematologic and solid malignancies. Contrary to the patient with HIV infection, cavitation is extremely rare in oncology patients (Figure 19). Diffuse bilateral ground-glass attenuation is the most common finding in cancer patients.92
Pulmonary nocardiosis is an important cause of opportunistic infection in immunosuppressed patients, and the incidence of this infection is increasing. Solitary or multiple nodules with cavitation are detected in ≤80% of cases (Figure 20).93 Cavitation may be more frequently observed among patients with advanced HIV than among other hosts.94
Post therapy with novel chemotherapy agents
Novel chemotherapeutic agents, such as anti-angiogenic drugs, have been associated with cavitation in lung tumors. Bevacizumab (Avastin, Genentech, San Francisco, CA) is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF). Bevacizumab has been associated with cavitation in primary and metastatic lung cancer. Cavitation is secondary to central necrosis due to VEGF inhibition and signifies therapeutic response. Spontaneous pneumothorax has been reported when a responding cavitary lesion communicates with the pleura (Figure 21).95
Post radiation therapy
Radiation therapy is an important technique in treating cancer. Radiation-induced injury in the lung is dependent on radiation dose, fractionation of dose, portal size and concurrent or prior administration of chemotherapy.96 In the early stages, radiation pneumonitis may present as ground-glass opacification confined to the radiation port. Cavitation may be seen 6 to 12 weeks post radiation, secondary to necrosis and expulsion of necrotic components. Delayed complications can include spontaneous pneumothorax, secondary to rupture of bleb or ectatic bronchus to the pleura, and development of bronchopleural fistula, secondary to superimposed infection on a previously radiated lung parenchyma (Figures 22 and 23).97
Post radiofrequency ablation
Radiofrequency ablation (RFA) has been advocated as a safe and minimally invasive procedure for patients with lung tumors. Cavitation has been described as evolution of post ablation changes in treated lesions after RFA therapy (Figure 24).94 Cavitation usually occurs within the tumor between 1 to 3 months following RFA and evolves over time.94 The frequency of cavitation is reported to be significantly higher in patients with lung cancer as the primary lesion, for lesions located within 1 cm of the chest wall, and for pulmonary emphysema.94
Conclusion
Differentiation between malignant cavitary lesion and nonmalignant lesions in oncology patients can be challenging. Radiological findings can sometimes help in narrowing differential diagnosis; however, a comprehensive approach including symptoms and other clinical data is often required to obtain accurate diagnosis.
REFERENCES
- Tuddenham WJ. Glossary of terms for thoracic radiology: Recommendations of the Nomenclature Committee of the Fleischner Society.AJR Am J Roentgenol. 1984;143:509-517.
- Dodd GD, Boyle JJ. Excavating pulmonary metastases. Am J Roentgenol Radium Ther Nucl Med. 1961; 85:277-293.
- Chiu FT. Cavitation in lung cancers. Aust N Z J Med. 1975;5:523-530.
- Chaudhuri MR. Primary pulmonary cavitating carcinomas. Thorax. 1973;28:354-366.
- Felson B, Wiot JF. Some less familiar roentgen manifestations of carcinoma of the lung. Semin Roentgenol. 1977;12:187-206.
- Miller RR, McGregor DH. Hemorrhage from carcinoma of the lung. Cancer. 1980;46:200-205.
- Mouroux J, Padovani B, Elkaïm D, Richelme H. Should cavitated bronchopulmonary cancers be considered a separate entity? Ann Thorac Surg. 1996;61:530-532.
- Onn A, Choe DH, Herbst RS, et al. Tumor cavitation in stage I non-small cell lung cancer: epidermal growth factor receptor expression and prediction of poor outcome. Radiology.2005;237:342-347.
- Vourtsi A, Gouliamos A, Moulopoulos L, et al. CT appearance of solitary and multiple cystic and cavitary lung lesions. Eur Radiol. 2001;11:612-622.
- Weisbrod GL, Towers MJ, Chamberlain DW, et al. Thin-walled cystic lesions in bronchioalveolar carcinoma. Radiology. 1992;185:401-405.
- Weisbrod GL, Chamberlain D, Herman SJ. Cystic change (pseudocavitation) associated with bronchioloalveolar carcinoma: A report of four patients. J Thorac Imaging.1995;10:106-111.
- Görich J, Gamroth A, Beyer-Enke S, et al. Differential computed tomographic diagnosis of cavity-forming space-occupying lesions of the lung. Rofo. 1987;147:479-485.
- Laurens RG, Jr, Pine JR, Honig EG. Spontaneous pneumothorax in primary cavitating lung carcinoma. Radiology. 1983;146:295-293.
- Kolodziejski LS, Dyczek S, Duda K, et al. Cavitated tumor as a clinical subentity in squamous-cell lung cancer patients. Neoplasma. 2003;50:66-73.
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: Diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135: 1269-1271.
- Woodring JH, Fried AM. Significance of wall thickness in solitary cavities of the lung: A follow-up study. AJR Am J Roentgenol. 1983;140:473-474.
- Honda O, Tsubamoto M, Inoue A, et al. Pulmonary cavitary nodules on computed tomography: Differentiation of malignancy and benignancy.J Comput Assist Tomogr.2007;31:943-949.
- Abramson S. The air crescent sign. Radiology. 2001;218:230-232.
- Wang LF, Chu H, Chen YM, Perng RP. Adenocarcinoma of the lung presenting as a mycetoma with an air crescent sign. Chest. 2007;131:1239-1242.
- Shuji, B, Jiro, F,Yoko, F, et al. Cavitary lung cancer with an aspergilloma-like shadow. Lung Cancer. 1999;26:195-198.
- Torpoco JO, Yousuffuddin M, Pate JW. Aspergilloma within a malignant pulmonarycavity. Chest. 1976;69:561-563.
- Liao WY, Liaw YS, Wang HC, et al. Bacteriology of infected cavitating lung tumor.Am J Respir Crit Care Med. 2000;161:1750-1753.
- Kim YI, Goo JM, Kim HY, et al. Coexisting bronchogenic carcinoma and pulmonary tuberculosis in the same lobe: Radiologic findings and clinical significance. Korean J Radiol.2001;2:138-144.
- Souilamas R, Danel C, Chauffour X, Riquet M. Lung cancer occurring with Mycobacterium xenopi and Aspergillus. Eur J Cardiothorac Surg. 2001; 20:211-213.
- Seo JB, Im JG, Goo JM, et al. Atypical pulmonary metastasis: Spectrum of radiologic findings. Radiographics. 2001;21:403-417.
- Godwin JD, Webb WR, Savoca CJ, et al. Multiple, thin-walled cystic lesions of the lung. AJR Am J Roentgenol. 1980;135:593-604.
- Fraser RG, Pare JAP. Diagnosis of diseases of the chest, Volume 2. 2nd edition. Philadelphia, PA: W.B. Saunders;1978:1132.
- Balikian JP, Herman PG. Non-Hodgkin lymphoma of the lungs. Radiology. 1979;132:569-576.
- Mondschein JF, Lazarus AA. Multiple bilateral upper lobe cavitary lesions in a patient with inguinal diffuse large cell lymphoma. Chest. 1993;103:583-584.
- Jackson SA, Tung KT, Mead GM. Multiple cavitating pulmonary lesions in non-Hodgkin’s lymphoma. Clin Radiol. 1994;49:883-885.
- Ray P,Antoine M, Mary-Krause M, et al. AIDS-related primary pulmonary lymphoma. Am J Respir Crit Care Med. 1998;158:1221-1229.
- Miller WT, MacGregor RR. Tuberculosis: Frequency of unusual radiographic findings. AJR Am J Roentgenol. 1978;130:867-875.
- Van Dyck P, Vanhoenacker FM, Van den Brande P, De Schepper AM. Imaging of pulmonary tuberculosis. Eur Radiol. 2003;13:1771-1785.
- Hadlock FP, Park SK, Awe RJ, Rivera M. Unusual radiographic findings in adult pulmonary tuberculosis. AJR Am J Roentgenol. 1980;134: 1015-1018.
- van Westerloo DJ, Knapp S, van’t Veer C, et al. Aspiration pneumonitis primes the host for an exaggerated inflammatory response during pneumonia. Crit Care Med. 2005;33:1770-1778.
- Winer-Muram HT, Rubin SA. Thoracic complications of tuberculosis. J Thorac Imaging. 1990;5: 46-63.
- McAdams HP, Erasmus J, Winter JA. Radiologic manifestations of pulmonary tuberculosis. Radiol Clin North Am. 1995;33:655-678.
- Rodrigo T, Caylà JA, García de Olalla P, et al. Characteristics of tuberculosis patients who generate secondary cases. Int J Tuberc Lung Dis. 1997;1: 352-357.
- Matsuoka S, Uchiyama K, Shima H, et al. Relationship between CT findings of pulmonary tuberculosis and the number of acid-fast bacilli on sputum smears. Clin Imaging.2004;28:119-123.
- Im JG, Itoh H, Shim YS, et al. Pulmonary tuberculosis: CT findings—early active disease and sequential change with antituberculous therapy.Radiology. 1993;186:653-660.
- Yamashita K, Matsunobe S, Tsuda T, et al. Solitary pulmonary nodule: Preliminary study of evaluation with incremental dynamic CT. Radiology. 1995;194:399-405.
- Kono R, Fujimoto K, Terasaki H, et al. Dynamic MRI of solitary pulmonary nodules: Comparison of enhancement patterns of malignant and benign small peripheral lung lesions. AJR Am J Roentgenol. 2007;188:26-36.
- Pitchenik AE, Rubinson HA. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis. 1985;131:393-396.
- Long R, Maycher B, Scalcini M, Manfreda J. The chest roentgenogram in pulmonary tuberculosis patients seropositive for human immunodeficiency virus type 1. Chest. 1991;99:123-127.
- Wang JY, Lee LN, Hsueh PR. Factors changing the manifestation of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2005;9:777-783.
- Ikezoe J, Takeuchi N, Johkoh T, et al. CT appearance of pulmonary tuberculosis in diabetic and immunocompromised patients: comparison with patients who had no underlying disease. AJR Am J Roentgenol. 1992;159:1175-1179.
- Lynch DA, Simone PM, Fox MA, et al. CT features of pulmonary Mycobacterium avium complex infection. J Comput Assist Tomogr. 1995;19:353-360.
- Miller WT, Jr. Spectrum of pulmonary nontuberculous mycobacterial infection. Radiology. 1994; 191:343-350.
- Albelda SM, Kern JA, Marinelli DL, Miller WT. Expanding spectrum of pulmonary disease caused by nontuberculous mycobacteria. Radiology. 1985;157:289-296.
- Hollings NP, Wells AU, Wilson R, Hansell DM. Comparative appearances of non-tuberculous mycobacteria species: A CT study. Eur Radiol. 2002;12:2211-2217.
- Christensen EE, Dietz GW, Ahn CH, et al. Radiographic manifestations of pulmonary Mycobacterium kansasii infections. AJR Am J Roentgenol. 1978;131:985-993.
- Maliwan N, Zvetina JR. Clinical features and follow up of 302 patients with Mycobacterium kansasii pulmonary infection: A 50 year experience. Postgrad Med J. 2005;81:530-533.
- Park Y, Kim TS, Yi CA, et al. Pulmonary cavitary mass containing a mural nodule: Differential diagnosis between intracavitary aspergilloma and cavitating lung cancer on contrast-enhanced computed tomography. Clin Radiol. 2007; 62:227-232.
- Chang WC, Tzao C, Hsu HH, et al. Pulmonary cryptococcosis: Comparison of clinical and radiographic characteristics in immunocompetent and immunocompromised patients. Chest. 2006;129: 333-340.
- Fox DL, Müller NL. Pulmonary cryptococcosis in immunocompetent patients: CT findings in 12 patients. AJR Am J Roentgenol. 2005;185:622-626.
- Nadrous HF, Antonios VS, Terrell CL, Ryu JH. Pulmonary cryptococcosis in nonimmunocompromised patients. Chest. 2003;124:2143-2147.
- Sheflin JR, Campbell JA, Thompson GP. Pulmonary blastomycosis: Findings on chest radiographs in 63 patients. AJR Am J Roentgenol. 1990;154:1177-11780.
- Kennedy CC, Limper AH. Redefining the clinical spectrum of chronic pulmonary histoplasmosis: A retrospective case series of 46 patients. Medicine (Baltimore). 2007;86:252-258.
- Greendyke WH, Resnick DL, Harvey WC. The varied roentgen manifestations of primary coccidioidomycosis. Am J Roentgenol Radium Ther Nucl Med. 1970;109:491-499.
- McAdams HP, Rosado de Christenson M, Strollo DC, Patz EF Jr. Pulmonary mucormycosis: Radiologic findings in 32 cases. AJR Am J Roentgenol. 1997;168:1541-1548.
- Conant EF, Wechsler RJ. Actinomycosis and nocardiosis of the lung. J Thorac Imaging. 1992;7:75-84.
- Cheon JE, Im JG, Kim MY, et al. Thoracic actinomycosis: CT findings. Radiology. 1998;209:229-233.
- Kwong JS, Müller NL, Godwin JD, et al. Thoracic actinomycosis: CT findings in eight patients. Radiology. 1992;183:189-192.
- Kim TS, Han J, Koh WJ, et al. Thoracic actinomycosis: CT features with histopathologic correlation. AJR Am J Roentgenol. 2006;186:225-231.
- Yano S, Usui N, Asai O, et al. Septic intramuscular embolism in a neutropenic patient with myelodysplastic syndrome accompanied by asymptomatic septic pulmonary emboli. Intern Med. 2005;44:1100-1102.
- Cook RJ, Ashton RW, Aughenbaugh GL, Ryu JH. Septic pulmonary embolism: Presenting features and clinical course of 14 patients. Chest. 2005;128:162-166.
- Huang RM, Naidich DP, Lubat E, et al. Septic pulmonary emboli: CT-radiographic correlation.AJR Am J Roentgenol. 1989;153:41-45.
- Murata K, Takahashi M, Mori M, et al. Pulmonary metastatic nodules: CT-pathologic correlation. Radiology. 1992;182:331–335.
- Dodd JD, Souza CA, Müller NL. High-resolution MDCT of pulmonary septic embolism: Evaluation of the feeding vessel sign. AJR Am J Roentgenol. 2006;187:623-629.
- Im JG, Han MC, Yu EJ, et al. Lobar bronchioloalveolar carcinoma: “Angiogram sign” on CT scans. Radiology. 1990;176:749-753.
- Bonomo L, Storto ML, Ciccotosto C, et al. Bronchioloalveolar carcinoma of the lung. Eur Radiol. 1998;8:996-1001.
- Kim TH, Kim SJ, Ryu YH, et al. Differential CT features of infectious pneumonia versus bronchioloalveolar carcinoma (BAC) mimicking pneumonia. Eur Radiol. 2006;16:1763-1768.
- Thomas E, Brewster DH, Black RJ, Macfarlane GJ. Risk of malignancy among patients with rheumatic conditions. Int J Cancer. 2000;88:497-502.
- Baruch AC, Steinbronn K, Sobonya R. Pulmonary adenocarcinomas associated with rheumatoid nodules: A case report and review of the literature. Arch Pathol Lab Med. 2005;129:104-106.
- Kuhlman JE, Hruban RH, Fishman EK. Wegener granulomatosis: CT features of parenchymal lung disease. J Comput Assist Tomogr. 1991;15:948-952.
- Lee KS, Kim TS, Fujimoto K, et al. Thoracic manifestation of Wegener’s granulomatosis: CT findings in 30 patients. Eur Radiol. 2003;13:43-51.
- Mayberry JP, Primack SL, Müller NL. Thoracic manifestations of systemic autoimmune diseases: Radiographic and high-resolution CT findings. Radiographics. 2000;20:1623-1635.
- Lohrmann C, Uhl M, Schaefer O, et al. Serial high-resolution computed tomography imaging in patients with Wegener granulomatosis: Differentiation between active inflammatory and chronic fibrotic lesions. Acta Radiol. 2005;46:484-491.
- Davies MJ, Hall DR. Wegener’s granulomatosis presenting as pulmonary metastases: Value of antineutrophil cytoplasmic antibodies in diagnosis. Respir Med. 1990;84:339-340.
- Uppal S, Saravanappa N, Davis JP, et al. Pulmonary Wegener’s granulomatosis misdiagnosed as malignancy.Br Med J. 2001;322:89-90.
- Ozaki S. ANCA-associated vasculitis: Diagnostic and therapeutic strategy. Allergol Int. 2007;56:87-96.
- Naumann R, Beuthien-Baumann B, Fischer R, et al. Simultaneous occurrence of Hodgkin’s lymphoma and eosinophilic granuloma: A potential pitfall in positron emission tomography imaging. Clin Lymphoma. 2002;3:121-124.
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: Spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21:1-7.
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol. 1986; 37:327-333.
- Lee AY, Levine MN. Venous thromboembolism and cancer: Risks and outcomes. Circulation.2003; 107:I17–I21.
- Curtis AM, Smith GJ, Ravin CE. Air crescent sign of invasive aspergillosis. Radiology. 1979;133:17-21.
- Gefter WB, Albelda SM, Talbot GH, et al. Invasive pulmonary aspergillosis and acute leukemia. Limitations in the diagnostic utility of the air crescent sign. Radiology. 1985;157:605-610.
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: Glossary of terms for thoracic imaging. Radiology. 2008;246:697-722.
- Reichenberger F, Habicht JM, Gratwohl A, Tamm M. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J. 2002;19:743-755.
- Pinto PS. The CT Halo Sign. Radiology. 2004;230:109-110.
- Bollée G, Sarfati C, Thiéry G, et al. Clinical picture of Pneumocystis jiroveci pneumonia in cancer patients. Chest. 2007;132:1305-1310.
- Yoon HK, Im JG, Ahn JM, Han MC. Pulmonary nocardiosis: CT findings. J Comput Assist Tomogr. 1995;19:52-55.
- Buckley JA, Padhani AR, Kuhlman JE. CT features of pulmonary nocardiosis. J Comput Assist Tomogr. 1995;19:726-732.
- Lee JM, Jin GY, Goldberg SN, et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: Preliminary report. Radiology. 2004;230:125-134.
- Sandler AB, Johnson DH, Brahmer J, et al. A study of clinical and radiographic risk factors associated with early onset severe pulmonary hemorrhage in bevacizumab (Avastin) treated patients with advanced non-small cell lung cancer. Proc Am Soc Clin Oncol. 2006;24:7068.
- JJ Fennessy. Irradiation damage to the lung. J of Thoracic Imaging. 1987;2:68-79.
- Frytak S, Lee RE, Pairolero PC, et al. Necrotic lung and bronchopleural fistula as complications of therapy in lung cancer. Cancer Invest. 1988;6:139-143.
- Okuma T, Matsuoka T,Yamamoto A, et al. Factors contributing to cavitation after CT-guided percutaneous radiofrequency ablation for lung tumors. J Vasc Interv Radiol. 2007;18:399-404.
Related Articles
Citation
Cavities in the Lung in Oncology Patients: Imaging Overview and Differential Diagnoses. Appl Radiol.
June 9, 2010