Cardiac CT in the emergency room
Images

Dr. Gopal is an Assistant Director, Cardiovascular CT Imaging, and Dr. Budoff is an Associate Professor of Medicine and the Fellowship Program Director, Division of Cardiology, the Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center, Torrance, CA. Dr. Akincioglu is an Assistant Professor, Department of Nuclear Medicine, The University of Western Ontario, London, ON, Canada.
The diagnosis of acute coronary syndrome is missed in 2% to 4% of cases and is associated with a twofold increase in mortality, which results in a low threshold for hospital admission of patients with chest pain presenting to the emergency room (ER). 1 More than 2 million patients with acute chest pain are unnecessarily admitted to the hospital each year in the United States. 1 The limited ability to make the correct triage decisions can also lead to resultant liability issues that account for 20% of ER malpractice dollar losses. 1,2
The prognostic value of variables such as patient age, sex, presence of risk factors, and biochemical markers for major adverse cardiovascular outcomes is limited. 1 Therefore, ER triage decisions based on estimates of acute coronary syndrome risk levels derived from various clinical predictors are often ineffective, particularly for patients who have chest pain but normal initial cardiac enzyme levels and normal or nondiagnostic electrocardiograms. 1 More than 5 million patients with acute chest pain present to the ER in the United States every year. 3 The current management methods do not permit an effective ER triage of patients with acute chest pain in whom initial troponin levels are not elevated and ischemic electrocardiographic (ECG) changes are not evident. 1
Until the advent of cardiac computed tomography (CT), there has been no noninvasive diagnostic tool available that provides morphologic information about the presence and severity of coronary artery disease (CAD). As a result, the confirmation or exclusion of acute coronary syndrome, particularly in patients with unstable angina pectoris, requires extensive testing, and this necessity may lead to unnecessary hospital admission or, possibly, a delay in necessary treatment. 1,4,5 Needless to say, early triage of these patients is important from a management standpoint, as patients who are at the highest risk for adverse outcomes derive the greatest benefit from prompt and appropriate treatment, and those patients at low risk may be discharged quickly and safely. 6,7 Cardiac CT with the use of both calcium scoring and/or CT angiography (CTA), has the potential to substantially improve the clinical care of patients with acute chest pain presenting to the ER. 1
Cardiac CT
Traditional CT imaging of the chest in the acute setting has been extremely valuable in the diagnosis of pulmonary embolism (PE) and aortic dissection but is unable to provide accurate or detailed information on cardiac structures because of limited temporal resolution and motion arti-fact. 8 Significant advances have been made in the field of cardiac imaging, particularly in the ability to view the coronary artery lumen with sufficient diagnostic accuracy. This is quite an accomplishment, as noninvasive coronary angiography has been challenging, given rapid cardiac motion, small and tortuous vessels, concomitant calcification, and overlying veins. These factors have required imaging modalities used for noninvasive coronary angiography to possess both high temporal and high spatial resolution.
Cardiac CT is now a robust technology for the noninvasive assessment of a spectrum of cardiovascular disease processes and is poised to become a noninvasive method to evaluate the lumen of the coronary arteries. One of the most important advances has been faster gantry rotation speed resulting in better temporal resolution and better z-axis spatial resolution made possible by thin collimations with extensive volumetric acquisition. 9 The new 64-detector multidetector computed tomographic (MDCT) scanners provide fast scan times, improved cardiac gating options, and isotropic resolution, which provides 3-dimensional (3D) information free of superimposed tissues or interference, resulting in uniform resolution throughout. The 64 detectors yield a 3D data set: for example, near isotropic voxels of 0.35 × 0.35 × 0.5 mm 3 that could be rotated in any given plane without loss of resolution.
Calcium scoring in the ER
The first major application validated with this imaging modality is the assessment of atherosclerotic plaque burden and CAD risk through coronary artery calcium (CAC) scoring. 10 Three studies have documented that CAC is a rapid and efficient screening tool for patients admitted to the ER with chest pain and nonspecific electrocardiograms. 11-13 These studies show sensitivities of 98% to 100% for identifying patients with acute myocardial infarction and long-term event rates approaching zero for patients with negative tests (calcium scores of zero). The high sensitivity and negative predictive value may allow early discharge of those patients with nondiagnostic ECG and negative CAC scans (scores = 0). The absence of calcium implies a very low rate (0.6%) of annual incidence of coronary events, and patients with a zero score and nondiagnostic ECG can be safely discharged from the ER. The limitation of calcium scoring is that there is no assessment of stenosis severity, and non-calcific plaque, albeit rare in the setting of a score of zero, may be missed. 12
Use of calcium scoring in risk stratification
Coronary artery calcium has been shown to be highly specific for coronary atherosclerosis and adds incremental prognostic data to traditional cardiac risk factors. 13 Coronary calcium scores (CCS) are easily obtained at the time of the CT. The absence of coronary calcium (CCS of 0) has an extremely high negative predictive value for the presence of obstructive CAD in patients older than 40 years. 14 Conversely, the presence of CAC in a symptomatic patient cohort is associated with adverse outcomes. There are situations, however, where obstructive atherosclerotic disease may exist with a low CCS, especially in younger patients with comorbidities such as diabetes. CT angiography in these patients may reveal the presence of noncalcified or "soft" plaques, reinforcing the need for aggressive medical therapy in this patient subset.
64-MDCT in the ER
The advent of 64-detector MDCT (64-MDCT) offers the clinician in the ER setting a powerful tool to evaluate acute cardiovascular disease, evaluating both calcific and noncalcific plaque. CT angiography using 64-MDCT can accurately evaluate a number of cardiac, pulmonary, and vascular pathologies. In addition to performing the so-called triple rule-out (to detect CAD, aortic dissection, and PE), CCT can evaluate other cardiac disease states that are relevant to the clinician who is making acute diagnoses. These disease states include deep venous thromboses (DVT), cardiomyopathies, and pericardial disease. This review will discuss the role of CCT in the evaluation of patients with acute cardiac disease and will propose an integrated algorithm for the work-up and risk stratification of these patients.
Triple rule-out
The possibility of scanning the whole chest in one acquisition to visualize the aorta, the coronary arteries, and the pulmonary arteries provides a new angle in the triage of patients with acute chest pain, since this will be a diagnostic test to evaluate for CAD, PE, and aortic dissection. Though this might be a possibility, there are some challenges the physician must consider. An attempt to slow the heart rate to <60 bpm for coronary CTA might not be physiologically suitable if the patient, in fact, has PE or congestive heart failure. Further, although intravenous beta blockade is generally viewed as prudent in acute myocardial infarction, a recent large study showed an increased risk of cardiogenic shock in this population, suggesting that the underlying hemodynamic condition must first be stabilized. 15
A single scan for both pulmonary and coronary applications is challenging. The timing of contrast must be optimized differently for PE evaluation (right heart filling) as compared with coronary artery and aortic assessment (left heart opacification). To visualize both pulmonary arteries and coronary arteries simultaneously, a long contrast infusion is necessary, allowing simultaneous opacification of the right- and left-sided structures of the heart. The coronary arteries will not be optimally visualized for interpretation in CTA that is performed to evaluate the presence of PE, as the bright contrast in the right ventricle and superior vena cava may obscure portions of the right coronary artery.
Two issues make it difficult for a single acquisition to encompass the coronary and pulmonary vasculature. The extended anatomy to be imaged is one consideration, the injection rate is another, and the acquisition timing a third. While we normally want the primary part of the bolus in the arterial phase in the heart for the coronary arterial system, we want the bolus earlier for the pulmonary arteries. Usual timing for a PE study is to inject 60 to 70 mL of contrast at 4 mL/sec and begin scanning when the contrast reaches the pulmonary outflow tract. The contrast typically reaches this point within 8 to 12 seconds from the start of the injection, and ECG triggering is normally not required for this examination. For optimal acquisition of the coronary arteries, a typical injection is 70 to 80 mL of contrast at 5 mL/sec followed by a 60-mL saline flush. The authors use bolus timing in this setting and begin scanning when the contrast is at equal density in the ascending aorta and in the pulmonary outflow tract. This typically occurs approximately 23 to 27 seconds after the start of injection.
For a triple rule-out examination, the difficulty is timing the contrast, taking into account the 10- to 12-second difference between the 2 phases. To accomplish this, either flow rates are slowed down or total contrast volume is greatly increased.
Concerns regarding radiation dose of this single acquisition have been raised. 16 If CCT with retrospective triggering is used from the apex to the base of the lung (to allow simultaneous acquisition of lung fields and coronary arteries), the effective radiation dose will be 25 to 40 millisieverts (mSv). Finally, the decision to order such tests must be based on a pre-test clinical probability of a particular disease process being present and preferably not by a "shotgun approach." Thus, there is a potential that this test will be ordered unnecessarily in the ER for "chest pain work-up." This leads to increased radiation exposure to the patient as well as added costs. Despite these concerns, it should be noted that a recent study found the feasibility of the triple rule-out protocol by injecting 120 mL of contrast at a rate of 4 mL/sec with a 20-second breathhold to allow for overlap and contrast enhancement of both systemic and pulmonary vasculatures. 17
A more prudent approach has been suggested. 16 This would entail performing CCT in the usual manner, with acquisition of the heart using retrospective gating, with a radiation dose between 12 and 15 mSv, with administration of 60 to 80 mL of contrast. Then, a CT to evaluate the pulmonary arteries (thicker slices, not retrospectively gated) can be performed, with approximately 40 mL of contrast and a radiation dose of 4 to 6 mSv. This allows for a reduction of radiation dose, requires no increase in contrast administration, and provides 2 optimal studies, instead of 1 suboptimal study.
Acute coronary syndrome
During risk stratification of a patient who is being evaluated for chest pain, the clinician is frequently confronted with nondiagnostic results, including a nondiagnostic ECG and a normal first troponin level. 18 Furthermore, waiting for the results of a second set of cardiac biomarkers can often delay diagnosis for several hours. In the Thrombolysis in Myocardial Infarction (TIMI) IIIB study, approximately 15% of a cohort in >1300 patients had an initial negative troponin with a later rise in cardiac biomarkers at 12 hours. 19
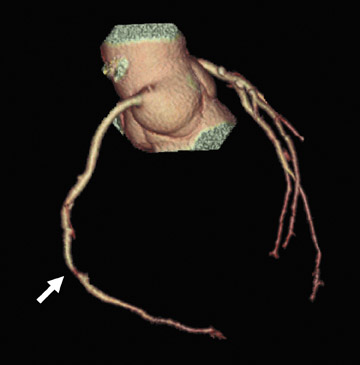
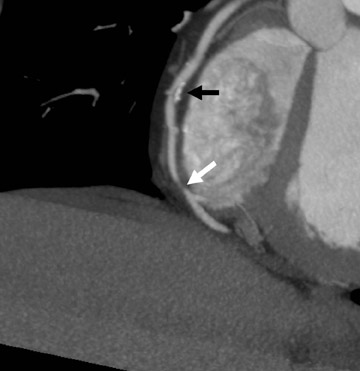
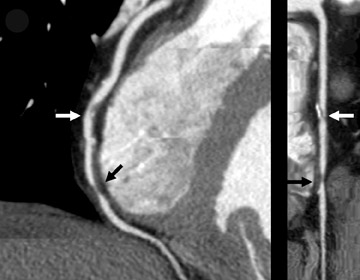
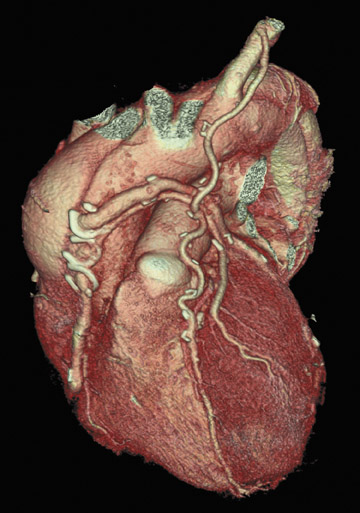
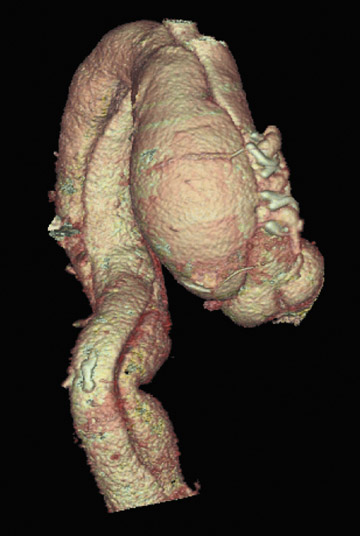
Perhaps the greatest benefit of 64-MDCT technology is the improved imaging quality of the coronary arteries and plaque assessment in the chest pain patient (Figures 1 through 3). In a recent study, the specificity and sensitivity for the detection of significant coronary stenosis (defined as >50% luminal narrowing) for 64-detector CTA of the coronary arteries compared with conventional coronary arteriography was 91% and 92%, respectively. 20 When compared with 16-MDCT, 64-MDCT offers faster scan times, fewer image artifacts, and better evaluation of distal vessels. Optimal image quality is obtained with slower heart rates (<65 bpm), which often requires the use of intravenous or oral beta blockade. The use of beta blockers, of course, may not be suitable in certain patients. A "positive" CTA for significant coronary artery or bypass graft stenosis would be of value to the interventional cardiologist, who can then focus on treating the culprit vessel in the cardiac catheterization laboratory without adding unnecessary fluoroscopy time. This is particularly helpful in patients who have anomalous coronary arteries and atypical anatomy of native coronaries and bypass grafts (Figure 4). Limitations of MDCT interpretation occur in calcified arterial segments and in segments with stents, which may cause a multitude of artifacts.
Gallagher et al 21 compared the accuracy of CTA with that of stress nuclear imaging for the detection of an acute coronary syndrome or 30day major adverse cardiac events (MACE) in low-risk chest pain patients. This was a prospective study of the diagnostic accuracy of myocardial perfusion imaging and MDCT in low-risk chest pain patients. The target condition was an acute coronary syndrome (confirmed >70% coronary stenosis on coronary artery catheterization) or MACE within 30 days. The patients were at low risk by the Reilly/Goldman criteria and had negative serial ECGs and cardiac markers. 21 All had both rest/stress technetium-99m sestamibi myocardial perfusion imaging and MDCT. Patients with abnormal stress nuclear imaging results (reversible perfusion defects) or MDCT results (stenosis >50% or calcium score >400) were considered for cardiac catheterization, and those with discordant results had a reevaluation (including ECG) by a cardiologist more than 30 days later. All patients were followed for evidence of MACE within 30 days by review of hospital records and structured telephone interviews.
Primary outcomes were the accuracy of MDCT and myocardial perfusion imaging for the detection of an acute coronary syndrome and 30-day MACE. Of the 92 patients, 7 (8%) were excluded because of uninterpretable MDCT scans. Of the remaining 85 study patients (median age 49 ± 11 years, 53% men), 7 (8%) were found to have the target condition, with all having significant coronary stenosis (88% ± 9%) and none having myocardial infarction or MACE during 30 days. Stress nuclear myocardial perfusion imaging results were negative in 72 (85%) patients, and MDCT results were negative in 73 (86%) patients. The sensitivity of stress nuclear imaging was 71% (95% confidence interval [CI] 36% to 92%), and MDCT was 86% (95% CI 49% to 97%), and the specificity was 90% (95% CI 81% to 95%) and 92% (95% CI 84% to 96%), respectively. The negative predictive value of stress nuclear imaging and MDCT was 97% (95% CI 90% to 99%) and 99% (95% CI 93% to 100%), respectively, and the positive predictive value was 38% (95% CI 18% to 64%) and 50% (95% CI 25% to 75%), respectively. The authors concluded that the accuracy of MDCT is at least as good as that of stress nuclear imaging for the detection and exclusion of an acute coronary syndrome in low-risk chest pain patients.
Acute aortic syndrome
Acute aortic syndrome (AAS) refers to the following life-threatening aortic emergencies: penetrating atherosclerotic ulcer, acute thoracic aortic injury, intramural hematoma, dissection, and aneurysm leakage. 22 MDCT offers several advantages over other imaging modalities in the diagnosis of acute aortic syndrome. Imaging can be obtained quickly and non-invasively. Other imaging modalities, such as transesophageal echocardiography, provide excellent imaging capability but are invasive and require an experienced operator who is readily available. This discussion will be limited to acute aortic dissection.
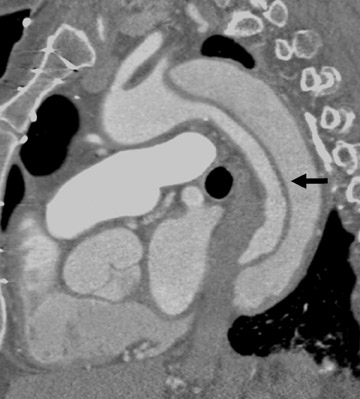
Acute aortic dissection is a potentially catastrophic emergency associated with high mortality. CT is a first-line diagnostic modality for the diagnosis. In a recent review that evaluated the accuracy of MDCT in acute aortic dissection, the authors reported a sensitivity of 99% and a specificity of 100% and concluded that CT is the test of choice, given its speed and cost-effectiveness. 23 The visualization of a dissection flap, of the true and false lumen, and of the origin and distal extent of the dissection are well characterized by CT (Figures 5 and 6). 24
Pulmonary embolism
The use of CT for the diagnosis of PE has been well established, initially with helical CT technology. 25 MDCT offers additional technical refinements and highly detailed visualization of the pulmonary vasculature. 26 The contemporary generation of scanners outperforms helical CT and has the added advantages of speed, ability to image sixth-generation vessels, and the capability to perform combined imaging of the lower extremity venous system for DVT. 27 The traditional belief that invasive pulmonary angiography is the "gold standard" for this diagnosis is under debate as a result of this technological improvement (Figure 7).
The combination of CT venography (CTV) and pulmonary angiography (CTVPA) was initially described in 1998 as a single comprehensive noninvasive imaging examination for suspected thromboembolic disease. 28 It allowed the identification of PE as well as DVT in the abdomen, pelvis, thighs, and calves. Combining CTV with CT pulmonary angiography (CTPA) not only increases the confidence in withholding treatment when results for both the pulmonary arteries and leg veins are negative but also increases the diagnosis of venous thromboembolism by 25% over CTPA alone. 29 The venographic portion of CTVPA has now been studied by multiple researchers and has been shown to be an accurate imaging study for the thigh veins in comparison with lower extremity sonography. In contrast to sonography, however, CTVPA readily and rapidly permits evaluation of the inferior vena cava, the pelvic veins, the calf veins, and all of the superficial venous system. Complex venous anatomy can be easily surveyed, and an additional sonographic study is not required. A review of 957 recent cases of suspected PE examined with CTVPA revealed an overall 10.5% frequency of DVT, with a nearly equal distribution of thrombosis at the common femoral, superficial femoral, popliteal, and deep calf veins. Although a variety of protocols for CTVPA may be implemented, including a contiguous helical acquisition, obtaining 5- or 10-mm-thick images every 4 cm provides a high degree of accuracy and decreases the overall radiation dose. 28 CT venography of the abdomen, pelvis, and lower extremities started 3 minutes after the start of contrast medium infusion for helical pulmonary CTA routinely produces high mean levels of venous enhancement. 30
In a prospective study, MDCT venography was compared with Doppler sonography in the detection of DVT of the pelvis and the thighs in patients with suspected PE. A total of 41 patients with suspected PE were included, and CTV (collimation 4 × 2.5 mm, table feed 12.5 mm, 120 kV, effective mAs 165) from the iliac crest to the knees was done after CTA of the pulmonary arteries. Doppler sonography was performed within 24 hours. Pulmonary embolism was diagnosed in 20 patients with additional DVT in 11 patients. The CTV had a sensitivity of 100%, a specificity of 96.6%, and positive and negative predictive values of 91.7% and 100%, respectively. The median cumulative effective dose for CTV was 8.26 mSv with a gonadal dose of 3.87 mSv. Changing the CTV protocol to a collimation of 4 × 5 mm with a 25-mm table feed reduced the dose by approximately 11% ( P <0.05) to 7.25 mSv and 3.35 mSv, respectively. The authors concluded that CTV is a safe and quick diagnostic tool for detecting DVT in patients with suspected PE. They also cautioned that because of the relevant increase in radiation dose, the need to perform the test has to be considered very carefully. 31
CT venography is as accurate as sonography in the diagnosis of femoropopliteal DVT. 32-35 CT venography can further reveal thrombus in large pelvis veins and the inferior vena cava, which is an important advantage over sonographic screening for DVT. 33 The iliac veins and vena cava are sometimes the source of significant PE emboli (in approximately 17% of patients with DVT). 34,35
A large multicenter study of PE was recently completed using both CTV and CTA. The use of CTA alone for the detection of PE had a sensitivity of 83% and a specificity of 96%. Adding CTV to CTA significantly increased the sensitivity to 90%. 36 This study mainly used 16-detector scanners. The expectation is that the use of 64-MDCT will improve sensitivity and provide very high negative predictive values (>98%).
An integrated approach to the chest pain patient
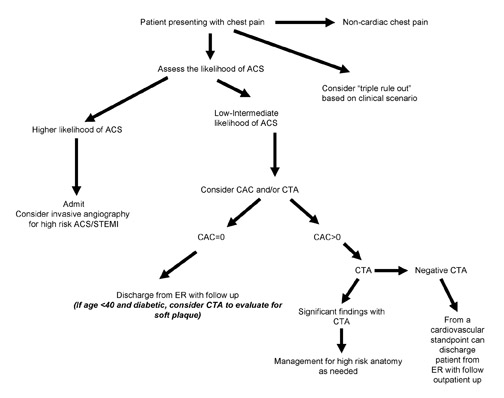
The use of CCT with 64-detector technology is a useful and powerful tool in the evaluation of the acute cardiac patient (Figure 8). The challenge remains in integrating this modality within the traditional work-up of cardiopulmonary disease. Many patients can be spared further invasive and costly testing with early risk stratification and imaging of a lower risk patient subset. It should be noted that patients presenting with noncardiac chest pain related to musculoskeletal or esophageal disease would be unlikely to benefit, diagnostically speaking, from CT imaging. Therefore, they require appropriate clinical management and would be candidates for early discharge and outpatient follow-up.
Limitations of cardiac CT
Current limitations of CCT of the native vessels include decreased resolution in obese patients and in those with markedly elevated CCS, the need for iodinated contrast and radiation exposure, and the need for reducing heart rate (typically with oral and/or intravenous beta-blocker administration) prior to evaluation with MDCT. 10 Ar-rhythmias or heart-rate variability compromise the ability to render 3D images from CTA data sets. An irregular heart rate is currently considered a contra-indication to CTA, and patients with irregular heart rates have been systematically excluded from most studies to date. 10 Other factors that interfere with the diagnostic quality or ability to perform CTA include difficulty with breath-holding, contrast allergies, and significant renal dysfunction. 10
Studies in patients with heart rates >70 bpm have had markedly reduced sensitivity and specificity because of motion artifacts. 10 In the study by Raff et al, 20 baseline heart rate at the time of the scan was the primary determinant for the accuracy of the study. When the heart rate was <70 bpm, the sensitivity was 97%, the specificity was 95%, the positive predictive value was 97%, and the negative predictive value was 95%. For patients with heart rates >70 bpm, the sensitivity declined to 88%, the specificity to 71%, the positive predictive value to 78%, and the negative predictive value to 83%. This study confirmed that patients with CCS >400, obesity (a body mass index ≥30 kg/m 2 ), and heart rates >70 bpm remain a challenge to diagnose with 64-detector CTA. 20
CT venography is specific but has a lower sensitivity rate and a lower positive predictive value for the diagnosis of acute lower extremity DVT compared with ultrasound. Additionally, CTV is more expensive than ultrasound scanning. Because of the lower sensitivity rate, the lower positive predictive value, and the increased cost of CTV, ultrasound remains the screening study of choice in cases of suspected acute lower extremity DVT. 37
Calcium scanning by either electron-beam CT or MDCT requires only a small radiation dose. The 0.6- to 1.2-mSv dose scan is similar to an abdominal X-ray and is at least half the radiation dose of invasive angiography. However, MDCT angiography imparts a significantly higher radiation dose to the patient (approximately 8 to 13 mSv). 38 With dose modulation (turning the X-ray beam down during systole when images are not as diagnostic due to motion), the dose is reduced by 18% to 47% depending on the patient's heart rate. 39 This moderate radiation dose must be considered when patients are selected for a CCT study. It must be kept in mind that the dose given during CTA is similar to that given during technetium-99m myocardial perfusion imaging protocols 40 ; therefore, this should not be considered excessive for the evaluation of CAD.
Conclusion
The use of cardiac CT in the emergency room, with its very high (99%) negative predictive power for obstructive coronary artery disease, PE, and aortic dissection, should allow for an expedited work-up of chest pain patients. This unprecedented accuracy will be attractive to ER physicians. However, the appropriate use of cardiac CT in the ER must be considered in each patient. While younger women on birth control pills with chest pain may have PE in the diagnosis, the likelihood of coronary artery disease and aortic dissection approaches zero. Thus, such patients should not get a complete "triple rule-out" study, with associated higher radiation doses and contrast requirements, but rather a targeted evaluation of the problem. Appropriate utilization will lower healthcare costs and reduce radiation exposure to patients. Limiting the use of this new technology to appropriate patients may prove harder than some CCT experts anticipate.
Related Articles
Citation
Cardiac CT in the emergency room. Appl Radiol.
November 12, 2007