Breast biopsy: Anesthesia, bleeding prevention, representative sampling, and rad-path concordance
Images


Chris I. Flowers, MD, FACR, is an Associate Professor of Oncological Science at the University of South Florida, Tampa, and the Director of Breast Imaging and Research at the Moffitt Cancer Center and Research Institute, WCB-RAD-MD, Tampa, FL.
Breast cancer is one of the most common cancers in the United States, killing an estimated 40,000 women annually, and was newly diagnosed in approximately 190,000 women in 2009, according to NCI SEER data (National Cancer Institute Surveillance Epidemiology and End Results).
To diagnose these cancers, at least 5 times this number will require a biopsy to determine whether a finding is benign or malignant. In the U.S. alone, this equates to approximately 1 million breast biopsies per year.
Nonpalpable cancers are usually picked up at a screening mammogram, and there has been a concomitant rise in the number of ductal carcinoma in situ diagnosed as a side effect of screening, which has caused some debate. Palpable findings, however, especially in younger women, occur frequently, and are usually the subject of image-guided biopsy to make the diagnosis.
As a result, biopsy is commonly used by breast imaging radiologists and focused on lesions falling into categories 4 (suspicious) and 5 (highly suspicious) of the BIRADS reporting system.1 Once the preserve of breast interventionalists, biopsy is now in the armamentarium of every practicing radiologist interpreting mammograms, and is a routine part of every practice.
In performing breast biopsy, the challenges we face on a daily basis are as follows:
- Ensuring adequate anesthesia;
- Preventing bleeding;
- Obtaining representative sampling of a lesion; and,
- Ensuring that we achieve radiological-pathological concordance.
We will discuss each of these issues and offer some pointers and practical advice in this article.
Adequate anesthesia
A patient told that she needs a biopsy suffers considerable anxiety, as most patients fear that they will be given a cancer diagnosis. There are even measurable systemic effects from the biopsy procedure.2 Therefore, to obtain a good quality biopsy, we need to ensure that the patient is comfortable during the procedure, being adequately anesthetized. This applies as much to stereotactic core biopsy as to magnetic resonance imaging (MRI) guided biopsy, both of which may take a prolonged time from start to finish.
Local anesthesia (LA) procedures using simple Lidocaine 1%, or combined with 1:100,000 Epinephrine (EPI) are identical to those of other radiological procedures.3-5 As LA is supplied as hydrochloride, and it must be nonionized to enter the nerve endings, it may be buffered with 8.4% sodium bicarbonate (Table 1). The resulting reduction in acidity also assists in reducing the ‘burn’ associated with the injection and improved patient comfort.
Another proven method of reducing the discomfort from the LA injection is to warm the Lidocaine to body temperature before injection.6-12
If using vacuum-assisted biopsy (VAB), due to the dead space of the needle distal to the sample notch, which may be up to 1 cm, at least 1.5 cm beyond the area being biopsied, should be anesthetized. In practice, this means using a long needle (eg, spinal needle) and intentionally injecting beyond the lesion. If you are performing a stereotactic biopsy, you can inject from the deepest part of the breast adjacent to the Bucky, all the way out to the skin.
Reduction of residual pain from deep VAB may be mitigated by a second LA injection.2 If you are using LA with EPI, avoid intradermal injection (to lessen the risk of skin necrosis).13 Sub-dermal injection does not have the same danger. LA combined with EPI has several advantages: It reduces bleeding by local vasoconstriction, prolongs anesthesia to approximately 6 hours, and reduces the systemic concentration, therefore making it rare for the patient to get toxic effects.
The syringe of LA can also be used for ‘blunt dissection’ – you can lift a mass off an implant/chest wall, or the skin, by injecting as you advance the needle slowly. Once you obtain a small, fluid-filled cavity, advance the needle while still injecting. This technique is similar to the surgical technique of blunt tissue dissection using forceps rather than a cutting blade.
Practice point: Use a long needle (eg, spinal needle) and intentionally inject beyond the lesion. If you are performing a stereotactic biopsy, you can inject from the deepest part of the breast adjacent to the Bucky, all the way out to the skin.
Difficult areas to anesthetize and the use of regional blocks
Some areas of the breast, in particular the immediate sub-areolar region, are difficult to anesthetize fully. As a result, if the patient then has a bad experience with her biopsy, she may never return for another mammogram.
An alternative technique, known as a regional anesthesia, is a ‘Nipple Block.’14 Your breast surgeon may be willing to teach this to you.
One of the most effective simple ways of achieving full anesthesia of the nipple areola complex (NAC) is to use topical Lidocaine, either as viscous Lidocaine or as a eutectic mixture of local anesthetics (EMLA, APP Pharmaceuticals) cream. Using EMLA 30 min prior to the procedure for a sub-areolar mass or a ductogram makes the process easier for yourself and the patient.
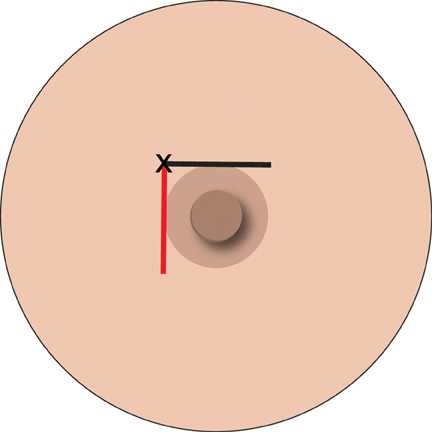
If adequate anesthesia has not been obtained there are two parts of the nipple block that can assist. The first is to inject in a stepwise manner, around the nipple areola margin (Figure 1).
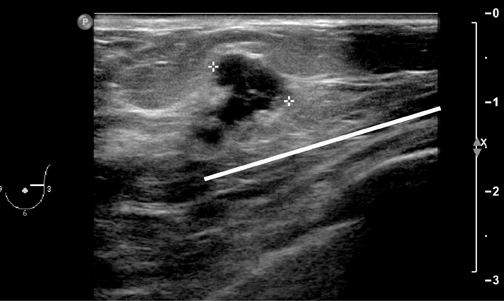
The second can achieve complete anesthesia, such that some surgeons can do a mastectomy. This requires the addition of a pool of 5 ml LA injected centrally in the breast, in the retro-glandular region. This takes out the penetrating branches of the intercostal nerves. This is easily achieved under ultrasound guidance from the lateral aspect of the breast (Figure 2).
Practice point: Good anesthesia is the key to a cooperative patient and a successful biopsy, but consider topical LA gels and regional block if the lesion is sub-areolar in position.
Prevention of bleeding: aspirin, warfarin, and clopidogrel
Any intervention in the breast may be complicated by hemorrhage/hematoma formation during, or following, the procedure. This is more common with the use of aspirin, warfarin (CoumadinTM) or clopidogrel (PlavixTM, Sanofi-Aventis, US).
The first confirmation that breast biopsy was safe to perform in patients on aspirin, heparin, or warfarin was published in 2000.15 This study was from an academic center, but it confirmed something that had been suspected for a long time.
The findings were validated in late 2008, when an article from community practice was published.16 This article showed that there was no significant risk of bleeding when patients are on aspirin or warfarin, and patients only need to be consented for an increased risk of hemorrhage/hematoma. A patient with a ‘therapeutic range’ INR, within 2 weeks of the procedure, is safe to biopsy. The only patients we still do not like to biopsy without withdrawal of therapy are those being treated with clopidogrel.
Practice point: Breast biopsy is safe in patients on antiplatelet or anticoagulant therapy, yet doubts still remain about patients on clopidogrel.
Representative sampling: Targeting and sampling error
One of the goals of a successful biopsy is to achieve adequate tissue sampling. This means that enough tissue is excised for pathological diagnosis. This is referred to as ‘representative’ sampling.
The choice of modality for biopsy and the size of the needle are important in obtaining a satisfactory tissue sample.
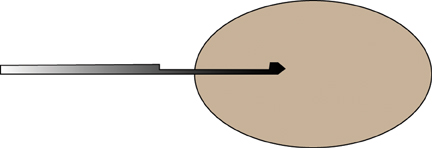
For ultrasound masses, it is often best to get a margin of a mass as part of the procedure, especially in the case of stromal sclerosis, fibrocystic change presenting as a mass, or hamartomas, as the central part of the mass may look like normal benign tissue under the microscope, whereas if a margin is included (Figure 3), then the pathologist may be able to identify a ‘mass,’ and report it as such.
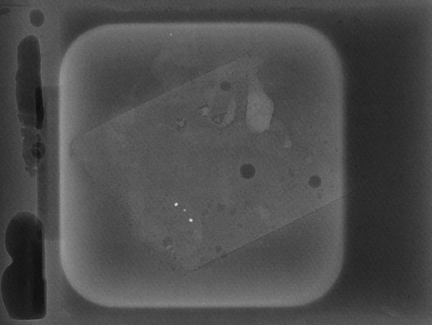
Similarly, calcifications are usually sampled by stereotaxis and a specimen x-ray is obtained to determine whether enough calcification was sampled to be representative of a lesion. If the calcified area is visible and biopsied under ultrasound guidance (Figure 4), then a specimen x-ray should be performed as if it had been performed under stereotaxis, to document removal of calcifications and representative sampling.
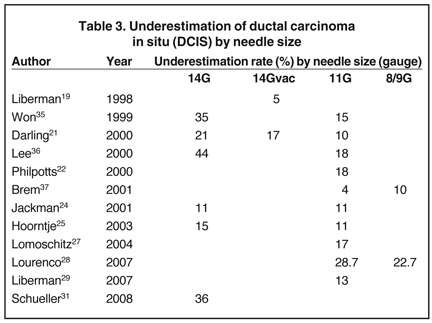
Needle size makes a difference when it comes to the issue of under-estimation of DCIS or invasive cancer. This is caused by not truly getting tissue that is a representative sample. Commonly, this occurs in a patient with suspicious calcifications and a core biopsy of atypical ductal hyperplasia (ADH), which eventually turns out to be DCIS.
Equally important is the underestimation of invasive disease, when only DCIS is found at core biopsy. We can reduce this variant of sampling error by targeting the asymmetric density/mass associated with suspicious calcifications (where there is an increased chance of finding invasive cancer at core).17, 18
Invasive disease is an important diagnosis to make pre-operatively, as the diagnosis of invasion mandates sentinel lymph node biopsy (SLNB), which otherwise is not required for ‘noninvasive’ disease.
Tables 2 and 3 demonstrate the differences between needle size and estimates of underestimation of ADH and ductal carcinoma in situ (DCIS) by publication.
Practice point: The larger the needle, the less under-estimation and, therefore, sampling error. It is important to note that a diagnosis of ADH currently requires surgical excision.38 Similar rates are found in patients having ultrasound or MRI-guided core biopsy.39-49
Sampling and unusual histological findings are as follows: The diagnosis of papilloma, and the role of subsequent surgical excision remain controversial. There is an even split in papers between excision and routine follow-up for women with a lesion compatible with a papilloma on imaging, with a pathology diagnosis of papilloma.50-61 However, the diagnosis of atypia or a papillary lesion always requires surgical excision.38
There are several other reasons for making a surgical excision following a core biopsy result. This may be to minimize false negative core biopsy or because of the need to excise surgically despite nonmalignant diagnosis at core biopsy. Additional reasons are as follows:
- Pathologist recommendation,
- Radiologic pathologic discordance,
- Histological underestimation, and,
- Unusual or ‘borderline’ histological lesions.
Some patients request that a palpable but proven benign lesion be surgically removed. This is one of the developing indications for the diagnostic excision of these lesions, either following a benign fine needle aspiration (FNA) or a core result.62-65 This is easily accomplished for lesions under 2 cm, with the reduction in costs associated with a fast outpatient procedure, and lesser morbidity (there is less visible scarring; therefore, it is useful in younger adults). Even if the fibroadenoma cannot be completely removed, the lesion has been debulked and is usually no longer palpable, and the patient can feel on the breast that the lump is gone, improving psychological outcomes.
Recurrence is possible if the lesion is more than 3 cm in size, but the procedure can be repeated without harm to the patient.65 The only exception (by size definition) is the juvenile fibroadenoma, which has a propensity to local recurrence, even with surgical excision.
Representative biopsy
Especially when performing a biopsy for calcifications, it is important to consider whether we have obtained a representative specimen.
Examine the specimen x-ray and compare it with the diagnostic images. We need to ensure that not only has calcium been harvested, but also whether the calcium harvested is representative of the lesion. Calcium then seen on histology can be correlated with that seen on the mammogram.
Practice point: A practice tip is to compare the specimen x-ray with the original prebiopsy diagnostic mammogram and determine whether you have calcium that represents what you see on the spot magnification films.
Concordance: Rad–path correlation
As the physician responsible for the biopsy, you are required to determine whether the pathological diagnosis reported to you fits the radiological findings. Another way of looking at it is to ask the question: Do the pathology results make sense in light of the clinical and imaging findings? If yes, there is concordance; if no, there is discordance. If there is doubt, it is always worth discussing with the pathologist, and even comparing what is seen under the microscope with the mammographic findings. One of the best ways to do this is to have a regular radiological-pathological meeting (Rad-Path) to discuss cases that are not straightforward. It is a good learning process for both parties.
Missing calcifications
How do you deal with a case of representative calcification sampling, seen on specimen x-ray, but a finding of ‘no calcifications seen’ on the pathology report?
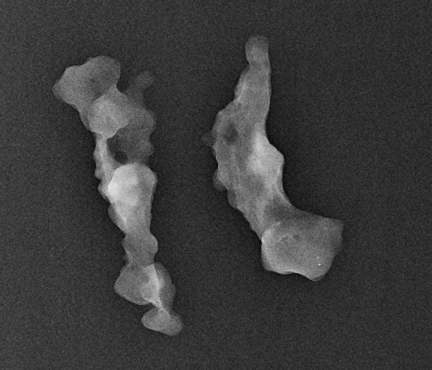
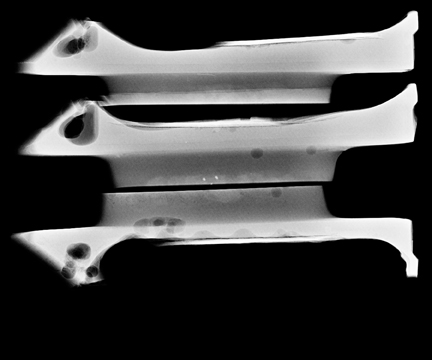
Ask to x-ray the specimen blocks if there are relatively few calcifications, or if the initial report is negative. This is best done in 2 planes—AP and lateral (Figure 5). A relatively high dose is required, using manual exposure, to get the best possible image quality. Once calcifications are identified, the blocks can then be returned to pathology with the specimen block x-rays, with details of which block contains the calcifications, and at what depth the calcifications are located in the block.
In Figure 5, the calcification is present but lies very superficially in one block. The pathologist must be informed that the calcification lies in the first 1/3 of the block, with one calcification particle at the anterior edge.
This is an important finding, as when the technician is making the slides from the blocks, the microtome shavings of the first few slices are discarded until a contiguous piece of tissue can be cut, and floated onto the slide. In this way, microcalcifications can be lost.66 Other reasons for calcifications going missing are:
- Microtome ‘knocked out’ calcifications (‘skid marks’ on slide), and
- Calcium oxalate crystals—best viewed under polarized light.67, 68
Practice point: If you have trouble with missing calcifications in pathology, x-ray the pathology blocks and check with polarized light for calcium oxalate.
Conclusion
In performing a breast biopsy, whether it be ultrasound, stereotactic or MRI-guided core biopsy, radiologists face challenges with ensuring adequate anesthesia, reduction of bleeding, representative sampling, and rad/path concordance. Practice points are provided to assist radiologists in applying the appropriate techniques, upon making a significant finding in biopsied lesions.
References
- American College of Radiology breast imaging reporting and data system atlas (BI-RADS Atlas). Reston, VA: American College of Radiology (ACR); 2003.
- Lang EV, Berbaum KS, Lutgendorf SK. Large-core breast biopsy: Abnormal salivary cortisol profiles associated with uncertainty of diagnosis. Radiology. 2009;250:631-637.
- Reynolds HE, Jackson VP, Musick BS. Preoperative needle localization in the breast: utility of local anesthesia. Radiology. 1993;187:503-505.
- Domeyer PJ, Sergentanis TN, Zagouri F, Zografos GC. Health-related quality of life in vacuum-assisted breast biopsy: Short-term effects, long-term effects and predictors. Health and Quality of Life Outcomes. 2010;8:11.
- Novy DM, Price M, Hunyh PT, Schuetz A. Percutaneous core biopsy of the breast: Correlates of anxiety. Academic Radiology. 2001;8:467-472.
- Martin S, Jones JS, Wynn BN: Does warming local anesthetic reduce the pain of subcutaneous injection? Am J Emerg Med. 1996;14:10-12.
- Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
- Cragg AH, Berbaum K, Smith TP. A prospective blinded trial of warm and cold lidocaine for intra-dermal injection. AJR Am J Roentgenol. 1988;150:1183-1184.
- Klein EJ, Shugerman RP, Leigh-Taylor K, et al. Buffered lidocaine: Analgesia for intravenous line placement in children. Pediatrics. 1995;95:709-712.
- Luhmann J, Hurt S, Shootman M, Kennedy R. A comparison of buffered lidocaine versus ELA-max before peripheral intravenous catheter insertions in children. Pediatrics. 2004; 113;3:217-220.
- Matsumoto AH, Reifsnyder AC, Hartwell GD, et al. Reducing the discomfort of lidocaine administration through pH buffering. J Vasc Interv Radiol. 1994; 5:171-175.
- Zagouri F, Sergentanis T, Gounaris A, et al. Pain in different methods of breast biopsy: Emphasis on vacuum-assisted breast biopsy. The Breast. 2008;17:71-75.
- Stoebner PE, Teot L, Colonna G, et al. Skin necrosis after ambulatory phlebectomy: Reconstructive surgery using an artificial dermis. Dermatologic Surgery. 2006;32:972-975.
- Kass R, Lind DS, Souba WW. ACS Surgery Section 3, Chapter 5 breast procedures. www.acssurgery.com/acs/chapters/ch0305.htm. Accessed January 25, 2010.
- Melotti MK, Berg WA. Core needle breast biopsy in patients undergoing anticoagulation therapy. AJR Am J Roentgenol. 2000;174:245-249.
- Somerville P, Seifert PJ, Destounis SV, et al. Anticoagulation and bleeding risk after core biopsy. AJR Am J Roentgenol. 2008;191:1194-1197.
- Bagnall MJC, Evans AJ, Wilson ARM, et al. Predicting invasion in mammographically detected microcalcification. Clinical Radiology. 2001;56:828-832.
- Huo L, Sniege N, Hunt KK, et al. Predictors of invasion in patients with core-needle biopsy-diagnosed ductal carcinoma in situ and recommendations for a selective approach to sentinel lymph node biopsy in ductal carcinoma in situ. Cancer. 2006;107:1760-1768.
- Liberman L, Smolkin JH, Dershaw D, et al. Calcification retrieval at stereotactic, 11- gauge, directional, vacuum-assisted breast biopsy. Radiology. 1998;208:251-260.
- Philpotts LE, Shaheen NA, Carter D, et al. Comparison of rebiopsy rates after stereotactic core needle biopsy of the breast with 11-gauge vacuum suction probe versus 14-gauge needle and automatic gun. AJR Am J Roentgenol. 1999;172:683-687.
- Darling ML, Smith DN, Lester SC, et al. Atypical ductal hyperplasia and ductal carcinoma in situ as revealed by large-core needle breast biopsy: Results of surgical excision. AJR Am J Roentgenol. 2000;75:1341-1346.
- . Philpotts LE, Lee CH, Horvath LJ, et al. Underestimation of breast cancer with 11-gauge vacuum suction biopsy. AJR Am J Roentgenol. 2000;175:1047-1050.
- Berg WA, Arnoldus CL, Teferra E, Bhargavan M. Biopsy of amorphous breast calcifications: Pathologic outcome and yield at stereotactic biopsy. Radiology. 2001;221:495-503.
- Jackman RJ, Burbank F, Parker S, et al. Stereotactic breast biopsy of nonpalpable lesions: Determinants of ductal carcinoma in situ underestimation rates. Radiology. 2001;218:497-502.
- Hoorntje LE, Peeters PH, Mali WP, Borel Rinkes IHM. Vacuum-assisted breast biopsy: A critical review. Eur J Cancer. 2003;39:1676-1683.
- Philpotts LE, Hooley RJ, Lee CH. Comparison of automated versus vacuum-assisted biopsy methods for sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2003;180:347-351.
- Lomoshitz FM, Heibich TH, Rudas M, et al. Stereotactic 11-gauge vacuum-assisted breast biopsy: Influence of number of specimens on diagnostic accuracy. Radiology. 2004;232:897-903.
- Lourenco AP, Mainiero MB, Lazarus E, et al. Stereotactic breast biopsy: Comparison of histologic underestimation rates with 11- and 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007;189:275-279.
- Liberman L, Holland AE, Marjan D, Murray MP, et al. Underestimation of atypical ductal hyperplasia at MRI-guided 9-gauge vacuum-assisted breast. AJR Am J Roentgenol. 2007;188:684-690.
- Sigalzafrani B, Muller K, Elkhoury C, et al. Vacuum-assisted large-core needle biopsy (VLNB) improves the management of patients with breast microcalcifications—Analysis of 1009 cases. European Journal of Surgical Oncology. 2008;34:377-381.
- Schueller G, Jaromi S, Ponhold L, et al. US-guided 14-gauge core-needle breast biopsy: Results of a validation study in 1352 cases. Radiology. 2008;248:406-413.
- Hoang J, Hill P, Cawson J. Can mammographic findings help discriminate between atypical ductal hyperplasia and ductal carcinoma in situ after needle core biopsy? The Breast. 2008;17:282-288.
- Youk JH, Kim EK, Kim MJ. Atypical ductal hyperplasia diagnosed at sonographically guided 14-gauge core needle biopsy of breast mass. AJR Am J Roentgenol. 2009;192:135-141.
- Eby PR, Ochsner JE, Demartini WB. Frequency and upgrade rates of atypical ductal hyperplasia diagnosed at stereotactic vacuum-assisted breast biopsy: 9-versus 11-gauge. AJR Am J Roentgenol. 2009;192:229-233.
- Won B, Reynolds HE, Lazaridis CL, Jackson VP. Stereotactic biopsy of ductal carcinoma in situ of the breast using an 11-gauge vacuum assisted device: Persistent underestimation of disease. AJR Am J Roentgenol. 1999;173:227–229.
- Lee CH, Carter D, Philpotts LE, et al. Ductal carcinoma in situ diagnosed with stereotactic core needle biopsy: Can invasion be predicted? Radiology. 2000;217:466-470.
- Brem RF, Schoonjans JM, Goodmas SN, et al. Nonpalpable breast cancer: Percutaneous diagnosis with 11- and 8-gauge stereotactic vacuum-assisted biopsy devices. Radiology. 2001;219:793-796.
- Jackman RJ, Birdwell RL, Ikeda DM. Atypical ductal hyperplasia: Can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology. 2002;224:548-554.
- Cardenosa G. Needle choice for sonographically guided core biopsy. AJR Am J Roentgenol. 1999;173:845- 846.
- Mainiero MB, Koelliker SL, Lazarus E, et al. Ultrasound-guided large-core needle biopsy of the breast: Frequency and results of repeat biopsy. J Women Imaging. 2002;4:52-57.
- Sauer G, Deissler H, Strunz K, et al. Ultrasound-guided large-core needle biopsies of breast lesions: Analysis of 962 cases to determine the number of samples for reliable tumour classification. Br J Cancer. 2005;92:231-235.
- Fishman JE, Milikowski C, Ramsinghani R, et al. US-guided core-needle biopsy of the breast: How many specimens are necessary? Radiology. 2003;226:779-782.
- Lee J, Kaplan JB, Murray MP, et al. Underestimation of DCIS at MRI-guided vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007;189:468-474.
- Jang M, Cho N, Moon WK, et al. Underestimation of atypical ductal hyperplasia at sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2008;191:1347-1351.
- Zagouri F, Sergentanis T, Koulocheri D, et al. Atypical ductal hyperplasia: A way to minimize underestimation in vacuum-assisted breast biopsy? The Breast. 2008;17;1-6.
- Renshaw, AA, Cartagena, M, Schenkman, RH, et al. Atypical ductal hyperplasia in breast core needle biopsies. Correlation of size of the lesion, complete removal of the lesion, and the incidence of carcinoma in follow-up biopsies. Am J Clin Pathol. 2001;116:92.
- Sneige, N, Lim, SC, Whitman, GJ, et al. Atypical ductal hyperplasia diagnosis by directional vacuum-assisted stereotactic biopsy of breast microcalcifications. Considerations for surgical excision. Am J Clin Pathol. 2003;119:248.
- Killebrew LK, Oneson RH. Comparison of the diagnostic accuracy of a vacuum-assisted percutaneous intact specimen sampling device to a vacuum-assisted core needle sampling device for breast biopsy: initial experience. The Breast Journal. 2006;12:302-308.
- Sigalzafrani B, Muller K, Elkhoury C, et al. Vacuum-assisted large-core needle biopsy (VLNB) improves the management of patients with breast microcalcifications – Analysis of 1009 cases. Eur J Surg Oncol. 2008;34:377-381.
- Page DL, Salhany KE, Jensen RA, Dupont WD. Subsequent breast carcinoma risk after biopsy with atypia in a breast papilloma. Cancer. 1996;78;258-266.
- Lewis JT, Hartmann LC, Vierkant RA et al. An analysis of breast cancer risk in women with single, multiple, and atypical papilloma. Am J Surg Pathol. 2006;30:665-672.
- Kraus FT, Neubecker RD. The differential diagnosis of papillary tumors of the breast. Cancer. 1962;15:444-455.
- Papotti M, Eusebi V, Gugliotta P, Bussolati G. Immunohistochemical analysis of benign and malignant papillary lesions of the breast. Am J Surg Pathol. 1983;7:451-461.
- Rabban JT, Koerner FC, Lerwill MF. Solid papillary ductal carcinoma in situ versus usual ductal hyperplasia in the breast: A potentially difficult distinction resolved by cytokeratin 5/6. Hum. Pathol. 2006;37:787-793.
- Liberman L, Bracero N, Vuolo MA, et al. Percutaneous large-core biopsy of papillary breast lesions. AJR Am. J. Roentgenol. 1999;172:331-337.
- Philpotts LE, Shaheen NA, Jain KS, et al. Uncommon high-risk lesions of the breast diagnosed at stereotactic core needle biopsy: Clinical importance. Radiology. 2000;216:831-837.
- Mercado CL, Hamele-Bena D, Singer C et al. Papillary lesions of the breast: evaluation with stereotactic directional vacuum- assisted biopsy. Radiology. 2001; 221:650-655.
- Mercado CL, Hamele-Bena D, Oken SM, et al. Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology. 2006;238:801–808.
- Sydnor MK, Wilson JD, Hijaz TA, et al. Underestimation of the presence of breast carcinoma in papillary lesions initially diagnosed at core-needle biopsy. Radiology. 2007;242:58-62.
- Shah VI, Flowers CI, Douglas-Jones AG, et al. Immunohistochemistry increases the accuracy of diagnosis of benign papillary lesions in breast core needle biopsy specimens. Histopathology. 2006;48:683-691.
- Liberman L, Tornos C, Huzjan R, et al. Is surgical excision warranted after benign, concordant diagnosis of papilloma at percutaneous breast biopsy? AJR Am J Roentgenol. 2006;186:1328-1334.
- Fine RE, Boyd BA, Whitworth PW, et al. Percutaneous removal of benign breast masses using a vacuum-assisted hand-held device with ultrasound guidance. Am J Surg. 2002;184:332.
- Tagaya N, Nagagawa A, Ishikawa Y, et al. Experience with ultrasonographically guided vacuum assisted resection of benign breast tumors. Clinical Radiology. 2008;63:396-400.
- Johnson A, Henry-Tillman R, Smith L, et al. Percutaneous excisional biopsy. Am J Surg. 2002;184:550–554.
- Grady I, Gorsuch H, Wilburn-Baily S. Long-Term Outcome of Benign Fibroadenomas Treated by Ultrasound-Guided Percutaneous Excision. The Breast Journal. 2008;14:275-278.
- Winston JS, Geradis J, Liu DF, Stomper PC. Microtome shaving radiography: Demonstration of loss of mammographic microcalcifications during histologic sectioning. The Breast Journal. 2004;10: 200-203.
- Tornos C, Silva A, El-Naggar A, Pritzker KP. Calcium oxalate crystals in breast biopsies: The missing calcifications. Am J Surg Pathology. 1990;14:961-968.
- D’Orsi C, Reale FR, Davis MA, Brown VJ. Is calcium oxalate an adequate explanation for non-visualization of breast specimen calcification. Radiology. 1992;182:801-803.