Abdominal Manifestations of Tuberous Sclerosis Complex
Introduction
Tuberous sclerosis complex (TSC) is a multisystem disorder that occurs 1 in 6000 live births.1 The condition’s manifestations are highly variable owing to penetrance, which determines the associated clinical presentation and outcome. Inherited in an autosomal-dominant fashion,2 TSC is caused by mutations or loss of function in 2 tumor suppressor genes, tuberous sclerosis complexes 1 and 2 ( TSC1 , TSC2 ). These genes encode hamartin and tuberin, respectively, and together with a third subunit, TBC1D7 , form a trimeric inhibitor complex that inhibits the mechanistic target of Rapamycin 1 (mTOR1) pathway, which is essential for normal growth.3 The loss of TSC1 or TSC2 results in constitutive action of the mTOR1 pathway, which eventually leads to abnormal production of the multisystemic hamartomatous tumors seen in TSC.4
Seizures and intellectual disability are the 2 most common clinical presentations of TSC.1 Dermatological findings are also common and present in 90% of cases.5 Imaging can play an important role in identifying affected organs and complications. Most of these studies focus on the neurological impacts of TSC, but imaging for thoracoabdominal manifestations is equally important. A retrospective study of patients in a TSC registry demonstrated that thoracoabdominal manifestations occur more frequently in these patients than in the general population, supporting the need for detailed thoracoabdominal imaging to better define disease extent.6 This review details the salient abdominal, pelvic, and chest manifestations of TSC to help facilitate and improve clinical diagnosis, treatment, and outcomes. The imaging features and complications of tuberous sclerosis lesions are summarized in Table 1 .
Tuberous Sclerosis Complex Manifestations
| Organ | Lesion | Imaging Features | Complications |
|---|---|---|---|
| Bone | Osseous lesions | Irregularly circumscribed and sclerotic appearance seen on CT. | Subperitoneal new bone deposition.1 Usually asymptomatic.7 |
| Heart | Rhabdomyoma | Variable signal intensities. Usually poorly enhancing. | Impaired ventricular function, valvular dysfunction, and outflow obstruction.8 |
| Kidney | AML (part of PEComa) | Loss of signal in fat-saturated and opposed phase images if typical AML. Lipid-poor AML shows scant fat and variable soft tissues of muscle and vessels with heterogeneous enhancement. T2 hypointense to isointense. | Hemorrhage if size > 4 cm or pseudoaneurysm > 0.5 cm.1, 9 Progression of chronic renal disease, renal failure.1 |
| Kidney | RCC | T2 hyperintense, heterogeneous to avid enhancement depending on subtype. | Hemorrhage, metastatic disease, venous thrombosis (bland and tumor), arterial involvement.10 |
| Kidney | Cysts | Cysts of varying complexities; can be fluid density, proteinaceous/hemorrhagic. | Chronic renal disease, renal failure, increased morbidity, cyst rupture. |
| Liver | AML (part of PEComa) | Similar to lipid-rich or lipid-poor AMLs in kidney. Lipid-poor hepatic AML can be confused with hepatocellular carcinomas and biopsy may be necessary to confirm. | Potential for rupture and hemorrhage11 but much less frequent than renal AMLs. |
| Lung/retroperitoneum | LAM (part of PEComa) | Thin-walled cysts. | Recurrent pneumothoraces and chylous pleural effusions or ascites.12 |
| Lung | MMPH | Ground glass nodules. | Usually stable disease. |
| Spleen | Hamartoma | Typically T1 hypointense, and T2 hyperintense. Small lesions may be isointense to the splenic parenchyma and seen as contour bulge. | Potential rupture, local mass effect.13 |
| Pancreas | AML (part of PEComa) | Typical pancreatic AML similar to those seen in the kidneys and liver. | May alter pancreatic function.14 Rare incidence. |
| Pancreas | Neuroendocrine tumor (rare) | Hypervascular and avidly enhancing. T2 hyperintense. Functional (hormone secreting) and nonfunctional tumors (non-hormone secreting). | Symptoms vary based on type of hormone secreted and if tumors are functional or not.15 |
| Multiorgan | Malignant PEComas | Larger size, heterogeneous enhancement, with areas of necrosis, can metastasize to distant organs and bones. | Metastatic disease, increased morbidity and mortality. |
Abbreviations : AML, angiomyolipoma; LAM, lymphangioleiomyomatosis; MMPH, micronodular pneumocyte hyperplasia; PEComa, perivascular epithelioid cell; RCC, renal cell carcinoma.
Abdominal/Pelvic Manifestations
Angiomyolipomas
Renal angiomyolipoma (AMLs) are the most common abdominal manifestation of TSC1 ; they are considered a subset of the perivascular epithelioid cell (PEComa) group of mesenchymal tumors. PEComas have varying growth potential, ranging from benign/nonaggressive entities such as AMLs, through lesions of uncertain malignant potential, to frankly malignant sarcomas that demonstrate aggressive behavior and metastatic potential.16 Malignant sarcomas will be discussed later in this review.
Renal AMLs are noted in 55-75% of individuals with TSC.13 They are characterized by abnormal blood vessels, immature smooth muscle cells, and fat cells.17 These entities are generally benign and asymptomatic, but large (typically >4 cm) AMLs and those with pseudoaneurysms >0.5 cm can predispose patients to an increased risk of hemorrhage.1, 9
Renal AMLs are classified by subtypes based on their fat content (lipid-rich and lipid-poor), which can be distinguished with imaging.18 MRI can assist in identifying lipid-rich AMLs owing to the presence of macroscopic fat, seen as areas of decreased signal intensity in frequency-selected fat-saturated MRI sequences ( Figure 1 ) and/or the presence of microscopic/intravoxel fat depicted as areas of decreased signal intensity and chemical shift type II artifact in opposed-phase MRI sequences ( Figure 1 ).18 On US, lipid-rich AMLs manifest as hyperechoic masses in the kidneys ( Figure 1 ); cross-sectional imaging is often utilized to confirm this finding and exclude fat-containing renal cell carcinoma (RCC).1 On CT, lipid-rich AMLs show macroscopic fatty lesions with attenuation < 10 HU.18 In addition, heterogeneous soft-tissue attenuation can also be seen on CT; contrast enhancement varies with vascularity and volume of soft tissue.18
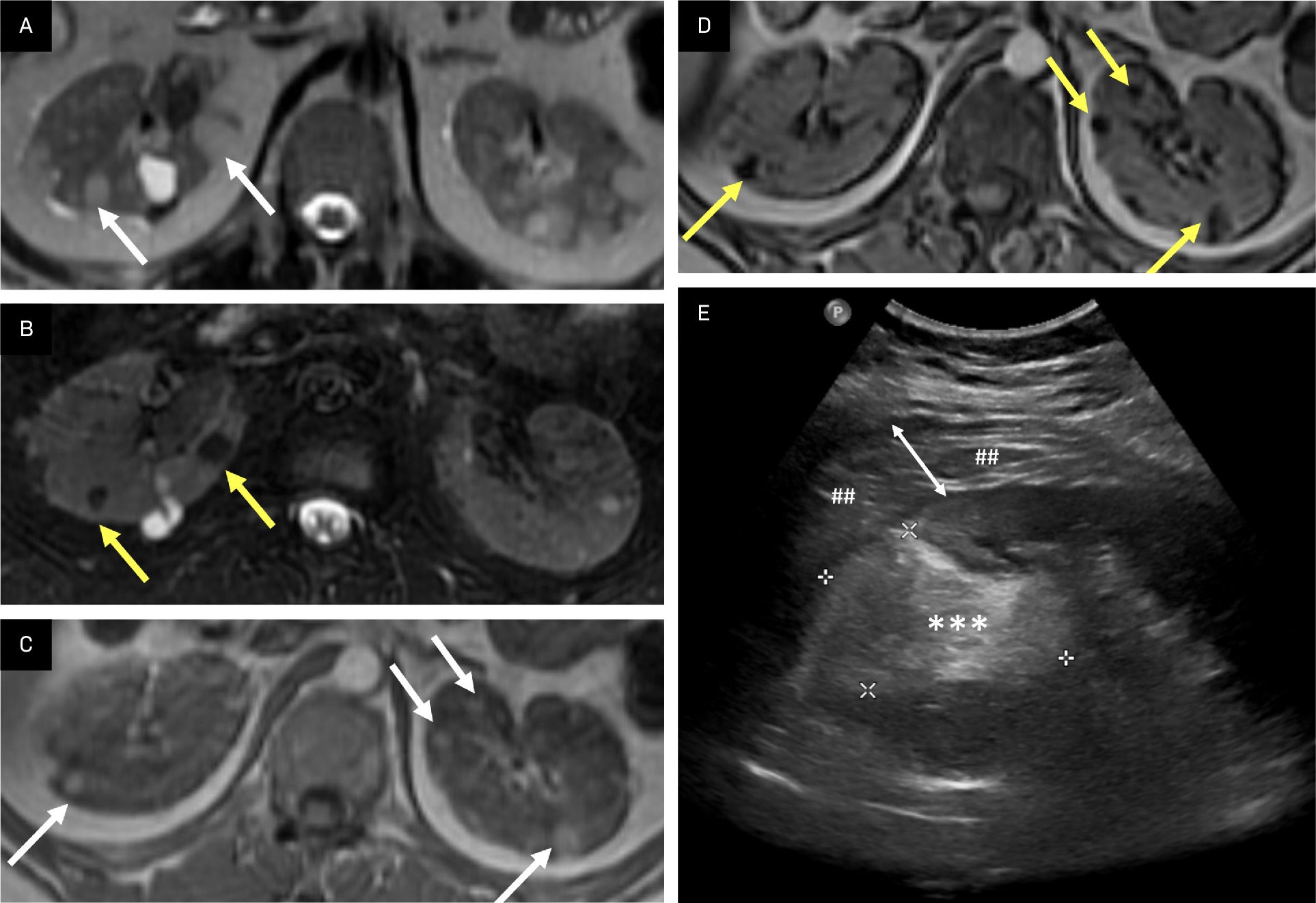
Lipid-rich angiomyolipoma (AML). AMLs typically contain macroscopic or bulk fat and can also contain microscopic or intravoxel fat. Intermediate lesions on T2 image (A, arrows) demonstrate loss of signal on T2-fat saturated image (B, arrows), indicating macroscopic fat. Phase imaging with T1 intermediate lesions in the kidneys (C, arrows) demonstrates loss of signal in the opposed phase image, indicating microscopic/intravoxel fat in AMLs (D, arrows). A large, left mid-to-superior renal pole echogenic lesion (E, asterisks and calipers) containing echogenic fat was found to be a ruptured AML with perinephric hematoma (##), whose thickness is delineated by the white double-headed arrow.
Lipid-poor AMLs are more complex, with variable soft-tissue composition, including muscular or vascular components with limited or absent fat where no significant loss of signal intensity is seen in the fat-saturated or opposed MRI sequences ( Figure 2 ).13 They are typically T2 isointense to renal parenchyma and demonstrate variable contrast enhancement patterns on MRI or CT ( Figure 3 ). Distinguishing lipid-poor AML from RCC can be challenging.1 The former appears to enhance variably and does not show the wash-in or wash-out characteristics typical of RCCs.10 Additionally, RCCs may contain microscopic and, rarely, macroscopic fat, thought to result from osseous metaplasia.19
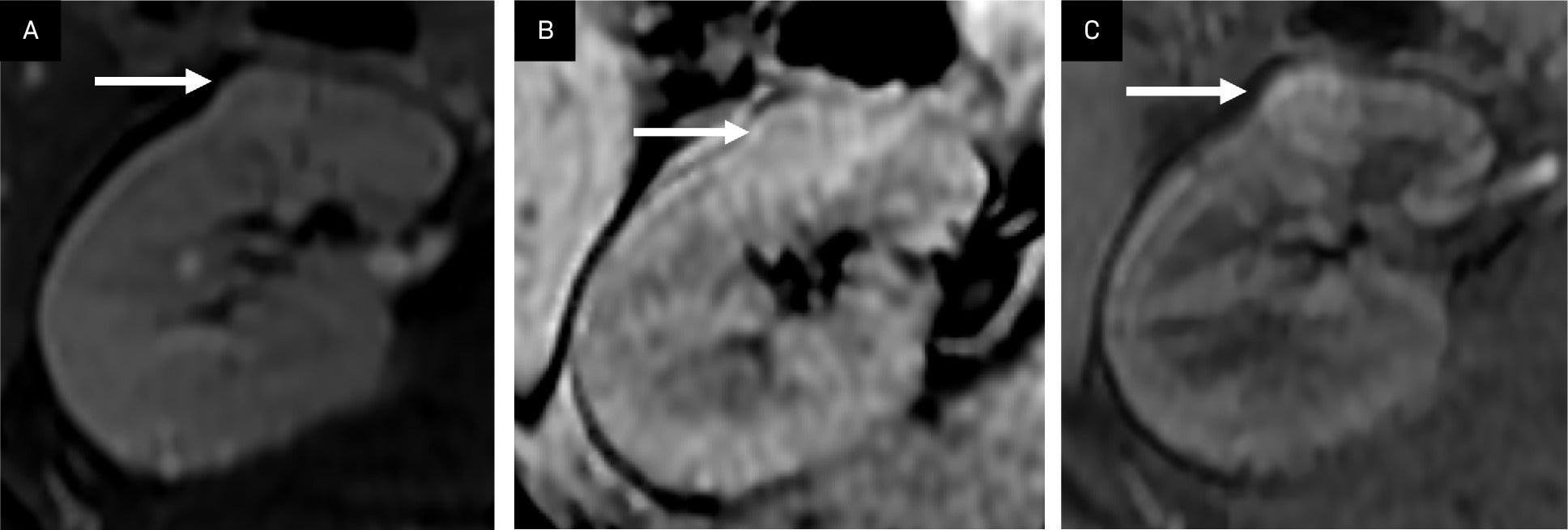
Lipid-poor angiomyolipoma (AML). A T2 isointense renal lesion without significant loss of signal in the frequency fat-saturated sequence (T2 FS) suggesting the presence of macroscopic fat (A, arrow), and no loss of signal in the T1 opposed phase sequence suggesting microscopic/intravoxel fat (B, arrow) demonstrates mild arterial enhancement in the T1 fat-saturated, contrast-enhanced image (C, arrow). This lesion can be difficult to distinguish from a renal cell carcinoma.
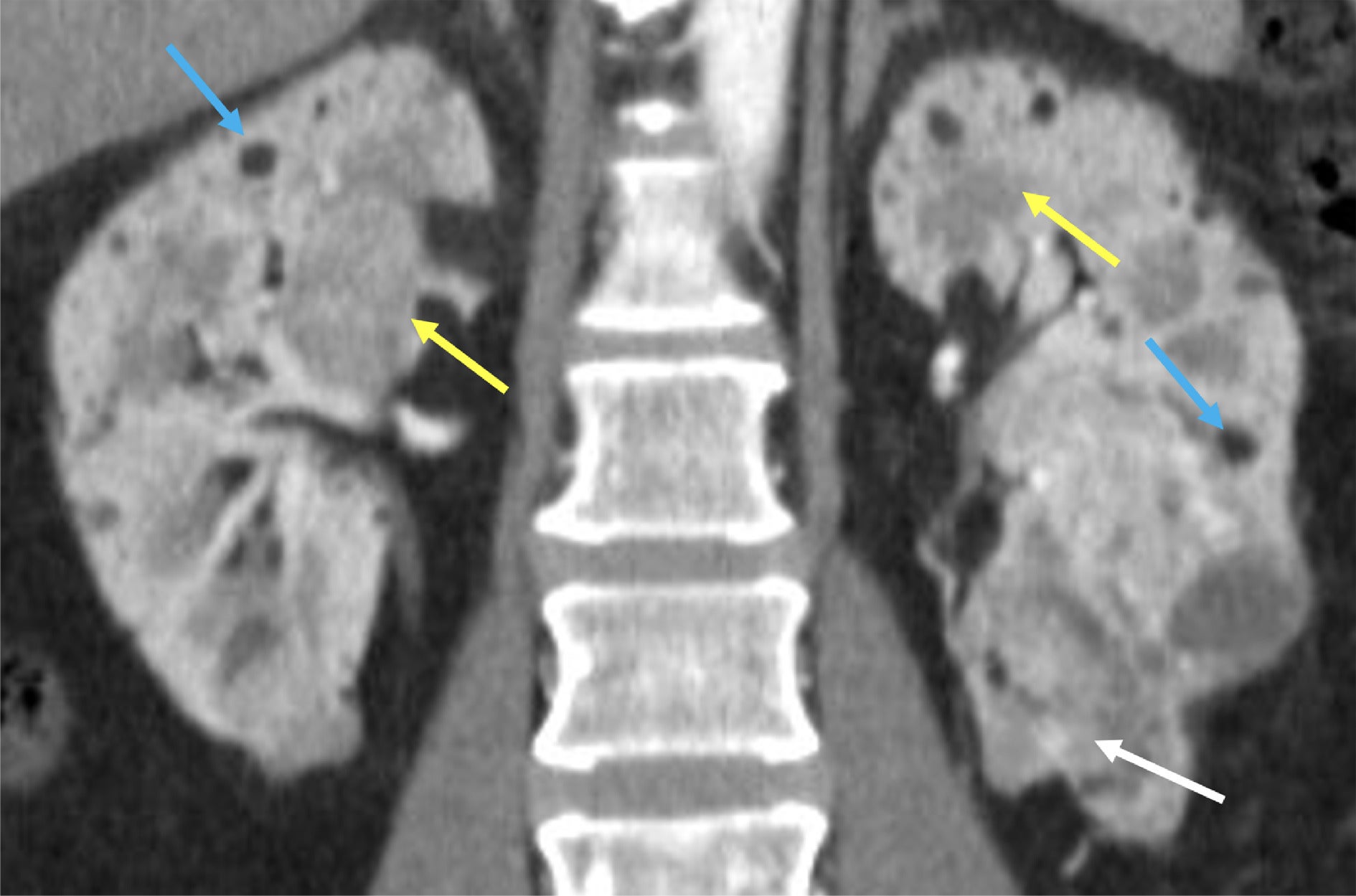
Variable appearances of renal angiomyolipomas (AMLs) in tuberous sclerosis complex. Predominantly macroscopic fat-containing AMLs show low CT attenuation with macroscopic fat <-10 HU (blue arrows). Lipid-poor AMLs with a more smooth muscle component are higher attenuation (yellow arrows). A more complex AML containing smooth muscle, vascular components, and tiny amounts of fat is seen in the left inferior renal pole (white arrow).
Although AMLs are usually benign, a rare subtype resulting from the transformation of a benign epithelioid AML via mutation can be malignant. These AMLs can present with locally invasive and aggressive features, as well as distant metastases known as multicentric AMLs of epithelioid morphology.13, 20 - 22 These lesions tend to be larger, appear more cellular, have internal necrosis and little fat, and demonstrate heterogeneous enhancement. Distant metastases may resemble RCCs on imaging, favoring a diagnosis of the more aggressive epithelioid AML over the typical benign AML.14, 23
In addition to the kidneys, AMLs can be found in other abdominal organs, though at a lower incidence. AMLs in the liver, pancreas, spleen, and/or gastrointestinal tract often coexist with renal AMLs.11, 24, 25 A study of 187 patients with TSC found that only 28 (14%) had hepatic AMLs with renal AMLs26 ; a large registry study similarly found that 19% of patients with TSC had liver AMLs.6 Hepatic AMLs can demonstrate a macroscopic fat component ( Figure 4 ) but may also be confused with hepatocellular carcinoma if they are lipid-poor. Biopsy is frequently required for confirmation.24 Hepatic AMLs are not typically stable lesions; they can grow and commonly demonstrate hypervascularity with macroscopic fat on contrast-enhanced imaging.13, 27 Unlike renal AMLs, hepatic AMLs rarely rupture, with only 8 such cases reported in the literature.28 These lesions tended to be large, with an average diameter of 8 cm. Hepatic AMLs > 5 cm with more aggressive epithelioid morphology on biopsy have been shown to benefit from surgical resection.29
Multiorgan angiomyolipomas (AMLs). Hepatic and pancreatic AMLs appear as regions of T2 hyperintensity in the liver and pancreas in T2. MR image (A, arrows) with loss of signal in the frequency selective fat-saturated T2 MR image (B, arrows).
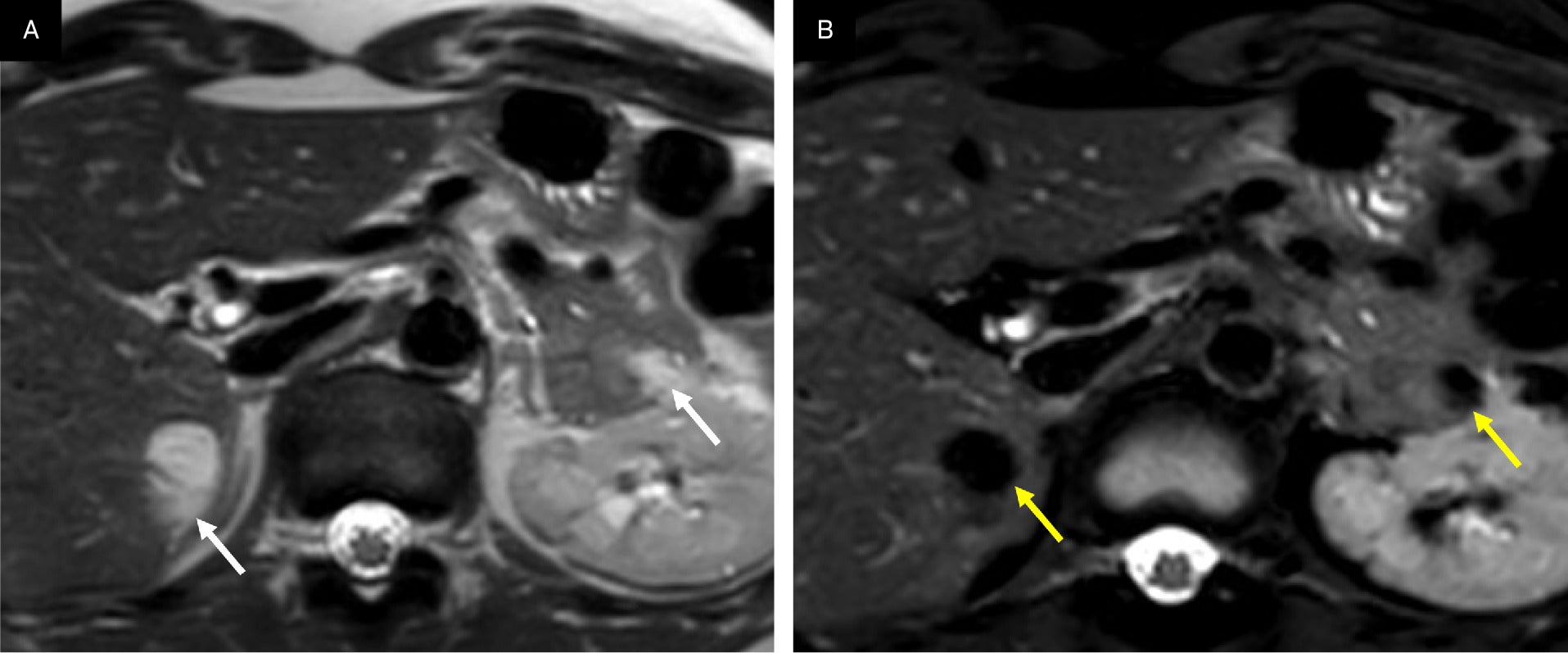
AMLs can also occur in the pancreas, where they manifest as macroscopic fatty lesions ( Figure 4 ) and may affect pancreatic function.11 These AMLs are extremely rare, with only a few cases reported,11, 30 and are among 1% of pancreatic tumors of mesenchymal origin.31 Heywood et al reported a case of a pancreatic AML with hemorrhage that appeared as a heterogeneous lesion on US and a cystic lesion with an irregular, thickened wall on CT.11
As alluded to earlier, large renal AMLs (>4 cm, Figure 5 ) or those that possess pseudoaneurysms > 0.5 cm can increase the risk for hemorrhage,1, 9, 32 which may be fatal if not detected and promptly treated. Manifestations of shock and hypovolemia in this setting are termed Wunderlich syndrome,32, 33 which, after status epilepticus, is the second-most frequent complication among patients with TSC.13 Prophylactic embolization or resection can help reduce mortality.1
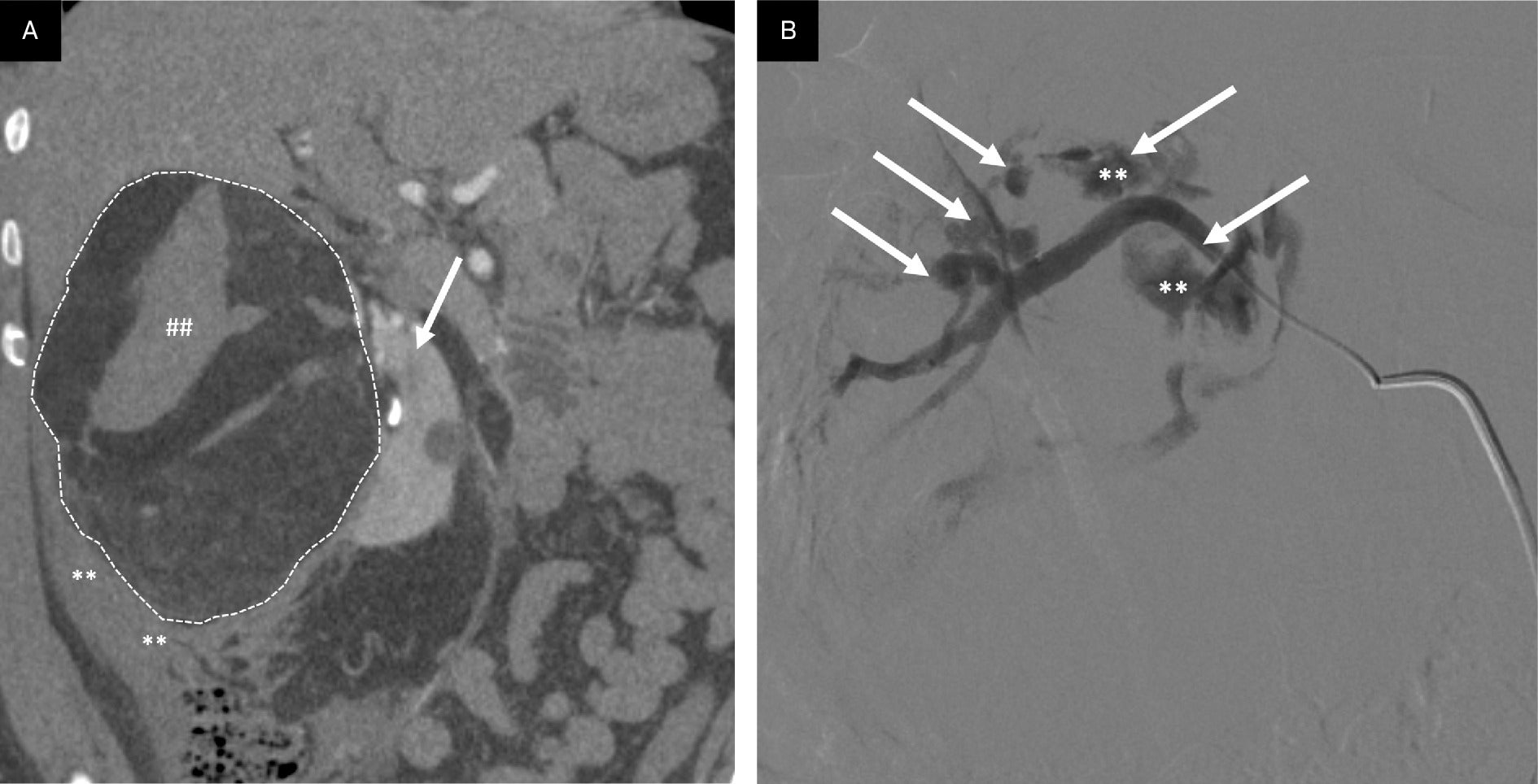
Angiomyolipoma (AML) rupture. AMLs > 4 cm or containing pseudoaneurysms > 0.5 cm have an increased propensity to bleed. (A) Coronal CT reformation through large, ruptured AML with macroscopic fat (dotted white lines outlining lesion) in the right kidney (arrow) with resultant perinephric hematoma (**) and internal hemorrhage (##). (B) Selective catheter arteriogram following AML rupture demonstrating multiple pseudoaneurysms (arrows), 2 with contrast extravasation indicating active hemorrhage (**).
Renal Cysts
Renal cysts and polycystic kidney disease are common in individuals with TSC ( Figure 6 )17, 34, 35 ; both were found in approximately 72% of patients in a large registry study.6 Renal cysts are typically asymptomatic but can lead to polycystic kidney disease and the development of hypertension and/or renal failure in the setting of TSC.17, 34, 35
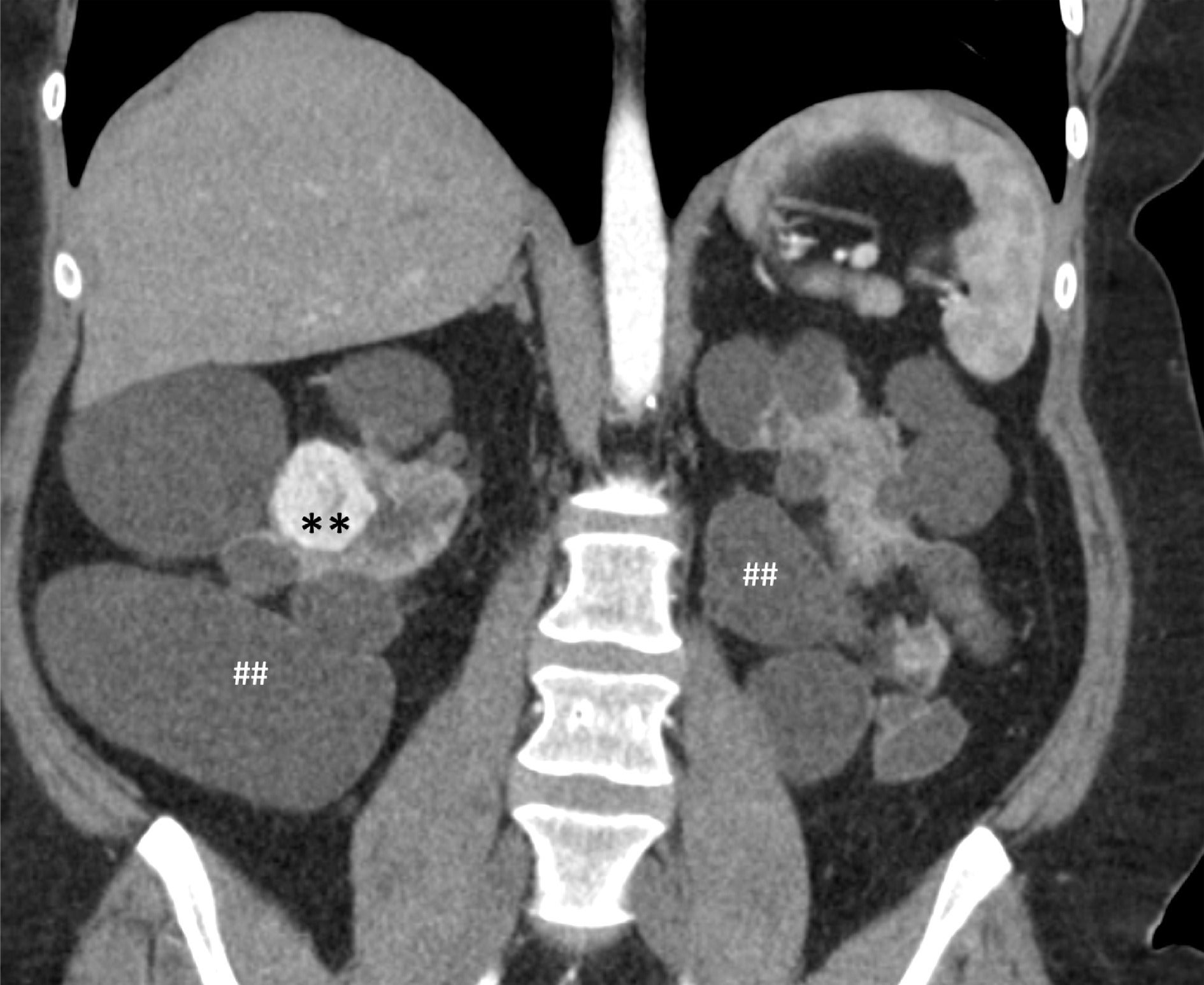
Renal cysts and renal cell carcinoma. Coronal CT reformation in a patient with tuberous sclerosis complex, end-stage renal disease, and associated polycystic kidney disease was found to have an avidly arterially enhancing right renal mass diagnosed as renal cell carcinoma (**) on a background of multiple renal cysts (##).
Renal Cell Carcinoma
Renal cell carcinoma is another manifestation that can occur in patients with TSC ( Figure 6 ).1, 8, 13, 36 - 39 These tumors tend to occur in young adults and can be of any subtype (clear cell, papillary, or chromophobe), the most common being clear cell.17, 38, 40 This finding underscores the importance of continuous imaging surveillance in patients with renal lesions, preferably with multiphasic MRI owing to its superior soft-tissue resolution, ability to perform chemical shift sequences and fat saturation, and lack of ionizing radiation. Multiphasic MRI should be performed every 1-3 years as part of a thoracoabdominal imaging protocol for TSC.41 Where MRI is contraindicated, multiphasic CT with a renal mass-oriented protocol may be helpful.
Hamartomas
Splenic hamartomas may be encountered in patients with TSC, albeit rarely.13 US imaging typically demonstrates solid masses hyperechoic to their surroundings along with increased blood flow on Doppler imaging.13 Other common sonographic findings are calcifications and cystic changes. On MRI, splenic hamartomas are usually T1 hypointense and T2 hyperintense to the splenic parenchyma. They can also be hypervascular and diffusely contrast-enhancing, particularly on early postcontrast imaging.13
Cardiothoracic and Musculoskeletal Manifestations
Lymphangioleiomyomatosis
One pulmonary manifestation of tuberous sclerosis is lymphangioleiomyomatosis (LAM),42 - 44 which is also a member of the PEComa group of tumors.16 LAM manifests as an abnormal proliferation of smooth muscle cells in the lymphatic system accompanied by cystic changes within the lung parenchyma. This can result in lung parenchymal destruction, dyspnea, and ultimately require lung transplantation.12, 45 There are 2 forms of LAM: one associated with TSC (TSC/LAM) and a more common sporadic form, both with female predominance.46 The TSC/LAM form is the more aggressive of the 2, presents with more severe disease, and is seen in 26-39% of patients with TSC.47 One complication of LAM is chylous pleural effusion formation, which disrupts the lymphatic system in the chest.1 On CT imaging, LAM will manifest as multiple thin-walled cysts ( Figure 7 ).44, 48 Reticular opacities may also be seen, which may indicate that edema is obstructing vessels.17 Pneumothorax is another complication of LAM that may require intervention.1, 17
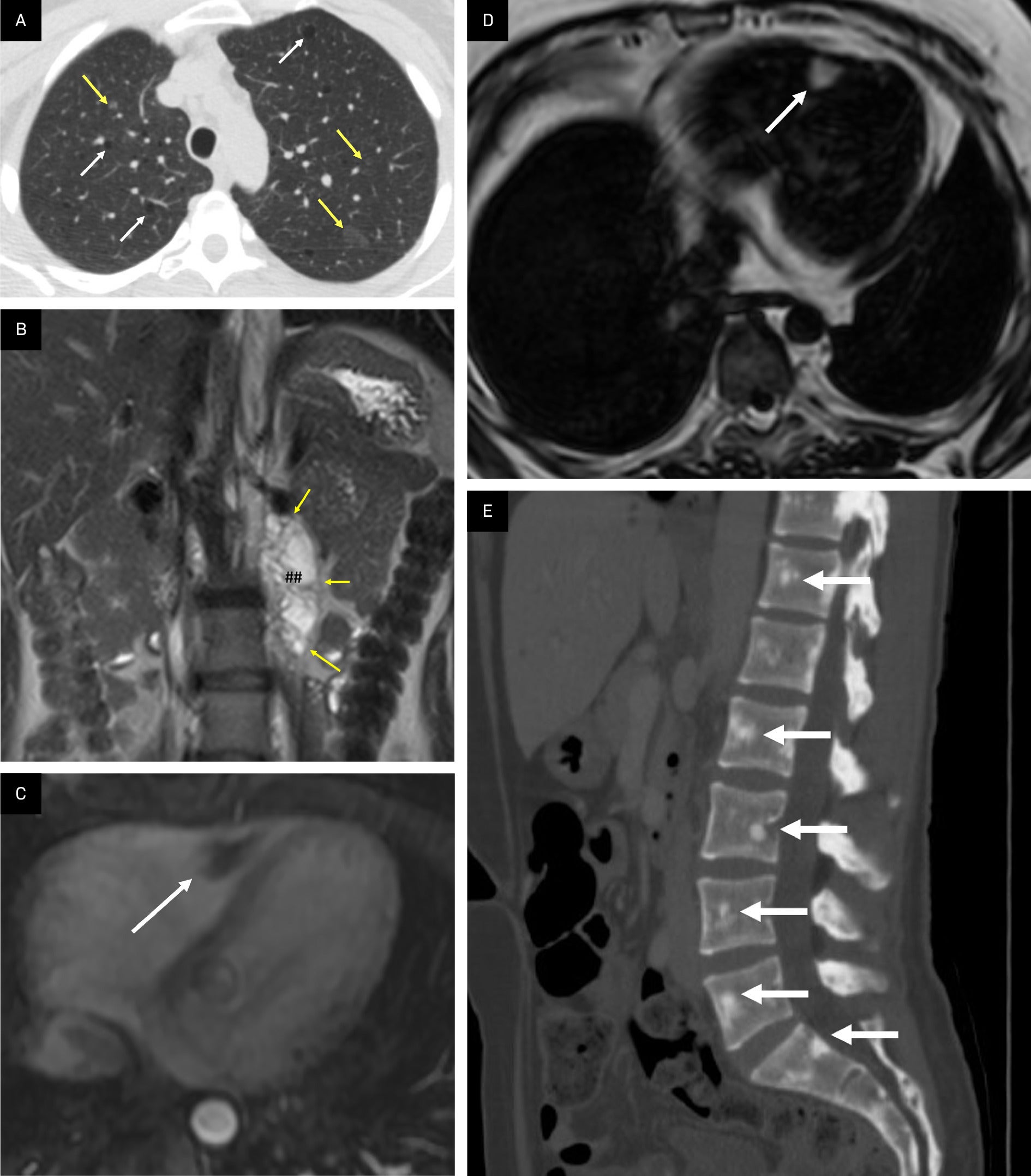
Other manifestations of tuberous sclerosis complex (TSC). (A) Axial CT showing pulmonary lymphangioleiomyomatosis characterized by thin-walled cysts (white arrows) and micronodular pneumocyte hyperplasia manifested by ground glass nodules (yellow arrows) in a patient with TSC. (B) Coronal T2 MRI showing how lymphangioleiomyomas can develop in the retroperitoneal region as in this T2 hyperintense signal mass (##, arrows) in the left retroperitoneal region abutting the left kidney. (C) T1 fat-saturated postcontrast axial image of the heart demonstrating a nonenhancing rhabdomyoma (white arrow) in the right ventricle. (D). Dixon fat-only MRI sequence with a tumor containing macroscopic fat at the ventricular septum (arrow) TSC appearing as a hyperintense lesion consistent with a cardiac angiomyolipoma. (E) Sagittal CT reformation of the spine in a patient with TSC and multiple sclerotic bony lesions in the vertebral bodies (arrows).
Lymphangioleiomyomas can also be seen in the retroperitoneal region as T2 hyperintense cystic dilatations involving the lymphatic system and lymphadenopathy.49 Complications include mass effect on retroperitoneal organs, chylous ascites, cyst rupture, and abdominal pain.7, 50
Micronodular Pneumocyte Hyperplasia
A third, less common, pulmonary manifestation of TSC is multifocal micronodular pneumocyte hyperplasia (MMPH), seen in approximately 18% of patients in a registry study.6 The condition is characterized by a well-demarcated nodular proliferation scattered randomly throughout the lungs and manifesting as ground glass nodules on CT ( Figure 7 ).49, 51 MMPH can occur concomitantly with or independently from LAM.
Cardiac Manifestations
Cardiac-related conditions are prominent in TSC.1, 38, 43 Approximately 70% of children with cardiac rhabdomyomas have TSC.1 Rhabdomyomas are benign, striated muscle tumors accompanied by “spider cells.”17, 42 US typically depicts a well-defined hyperechoic mass along the intraventricular septum.13 On MRI, rhabdomyomas show little to no contrast enhancement ( Figure 7 ).
Although less common than rhabdomyomas, AMLs can also be intracardiac and are usually located at the apex or in the ventricular wall.50 Noncontrast CT may show fat attenuation (<10 HU) in these lesions. Cardiac AMLs show macroscopic fat and are hyperintense on Dixon fat-only sequences ( Figure 7 ).
Osseous Manifestations
Osseous findings of TSC include sclerotic lesions, bone cysts, and periosteal new bone formation, the latter occurring particularly in the metacarpal areas.52 Sclerotic lesions can be identified in the ribs, vertebral posterior elements, and along the iliac side of the sacroiliac joints ( Figure 7 ).52 Rarely, TSC can cause macrodactyly.53 As nonspecific findings, these are nondiagnostic of TSC.52
PEComas
PEComas are rare mesenchymal neoplasms that can be benign or malignant entities with variable biologic features and oncologic outcomes.54, 55 Both types of lesions are associated with alterations predominantly of the TSC2 gene, while a distinct subset of malignant PEComas can harbor other mutations.55, 56 Benign lesions of the PEComa family include AML and LAM, while more aggressive entities termed malignant PEComas/sarcomas demonstrate a higher mitotic rate and growth potential. Malignant tumors can be found in patients with TSC and sporadically in patients without TSC.13, 15, 16
On imaging, malignant PEComas can be differentiated from their benign counterparts based on their typically larger size (>5 cm), infiltrative growth, necrosis, and/or osseous destruction ( Figure 8 ). They can also invade blood vessels and metastasize to other organs and tissues such as the liver, lungs, and bones.13, 15, 57 Owing to their deregulated growth, malignant PEComas can demonstrate fluorodeoxyglucose avidity on PET/CT.58 Multiphasic MRI and CT and PET/CT can all be useful in screening for malignant PEComas and assessing treatment efficacy with serial imaging studies.
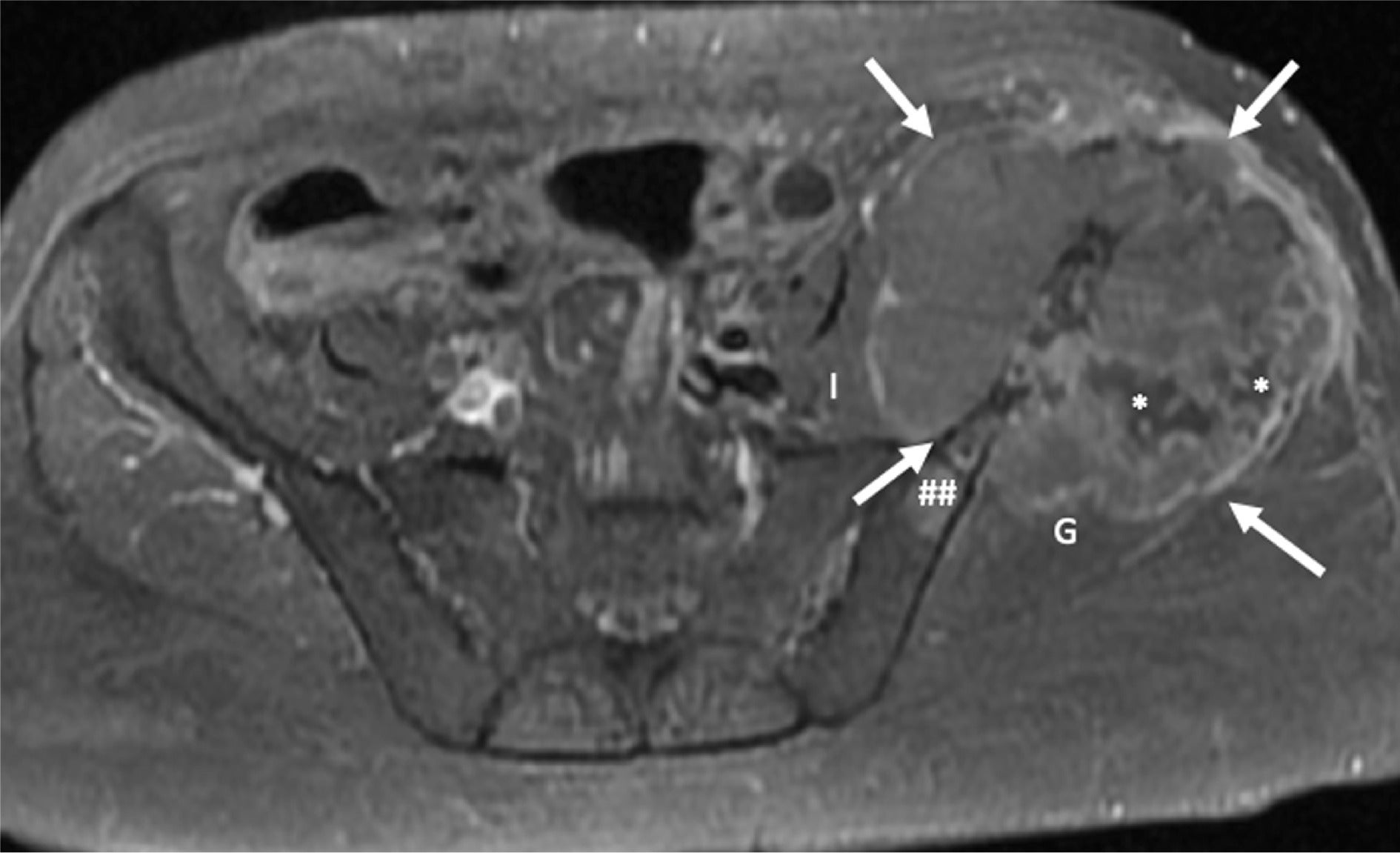
Aggressive malignant perivascular epithelioid cell tumor (PEComa). T1-fat saturated postcontrast MRI demonstrates an expansile and aggressive left pelvic lesion (arrows) eroding the left iliac crest and invading the adjacent left gluteal (G) and left iliopsoas (I) muscles. The tumor is heterogeneously enhanced with areas of internal necrosis (*) and was proven to be a recurrent malignant PEComa in a patient with a previous pulmonary PEComa that was treated and subsequently resected. A satellite lesion in the left iliac bone is also seen (##).
Patients with TSC with AMLs and non-AML PEComas, as well as some with gastrointestinal polyps, have an overall increased risk for developing malignancies. AMLs and non-AML PEComas are also associated with pancreatic neuroendocrine tumors,59 further underscoring the need for imaging surveillance.
Conclusion
Tuberous sclerosis complexhas a wide range of manifestations in the abdomen, pelvic, and chest. Renal AMLs are most common, but they can also occur in the liver, pancreas, and spleen. Thoracic manifestations include LAM, MMPH, and cardiac rhabdomyomas and AMLs. Recognizing these lesions can facilitate accurate diagnosis and optimal treatment planning in patients with TSC.
References
Citation
Akram MN, Xue K, Shiralkar KG, Chua SS.Abdominal Manifestations of Tuberous Sclerosis Complex. Appl Radiol. 2025; (2):21 - 30.
doi:10.37549/AR-D-24-0074
April 1, 2025