99mTc-Pyrophosphate Scintigraphy: History, Evolution, and Role in Cardiac Amyloidosis
Affiliations
- 1 Department of Radiology, University of Pennsylvania and Perelman School of Medicine, Philadelphia, Pennsylvania
- 2 Department of Radiology, University of Washington School of Medicine, Seattle, Washington
Cardiac amyloidosis is an important cause of heart failure, particularly among the elderly population. It manifests as restrictive cardiomyopathy resulting from myocardial infiltration of misfolded amyloid fibrils (light chain or transthyretin), leading to heart failure with preserved ejection fraction. The two main forms of cardiac amyloidosis are amyloid light chain and transthyretin-related (ATTR). There has been a notable increase in the prevalence of cardiac amyloidosis due to advances in diagnostic methods and effective treatment for ATTR amyloidosis after the discovery of tafamidis. We aim to discuss the interesting history and evolution of the pyrophosphate scan, which plays an important role in the diagnosis of ATTR amyloidosis.
Keywords: cardiac imaging, nuclear medicine
Introduction
The 99mTechnetium-pyrophosphate (99mTc-PYP) scan is a nuclear medicine imaging technique that is widely used for the diagnosis of cardiac amyloidosis. Its primary use has evolved from detecting acute myocardial infarction (AMI) to diagnosing cardiac amyloidosis, particularly transthyretin-related (ATTR) cardiac amyloidosis. Cardiac amyloidosis is a restrictive cardiomyopathy resulting from myocardial infiltration of misfolded amyloid fibrils (light chain or transthyretin), leading to heart failure. The two main forms of cardiac amyloidosis are amyloid light chain (AL) and transthyretin-related (ATTR). ATTR amyloidosis can be hereditary or wild type, which progresses slowly with advancing age.1 AL amyloidosis is rare (8-12 per million people per year) and caused by the accumulation of misfolded light chain proteins (amyloid fibrils) in the heart and different organs in plasma cell tumors such as multiple myeloma and carries a poor prognosis.2, 3 Wild-type ATTR (ATTRwt) is relatively more common, with its prevalence at 10-16% among elderly heart failure patients with or without aortic stenosis. Transthyretin (TTR) is a normal protein made by the liver; however, with advancing age, this can become unstable, misfold, and form amyloid deposits in various organs of the body, including the heart.4
Understanding the historical trajectory of the 99mTc-PYP scan reveals its scientific strengths, clinical utility, and the shifts in its role over the decades.
Early Cardiac Imaging and Myocardial Infarction Detection (1970s-1980s)
Originally introduced as a bone-imaging agent, 99mTc-PYP was among the earliest radiotracers used for cardiac imaging. In 1974, Parkey et al. found its utility in AMI, showing that PYP localizes to calcium deposits in infarcted myocardium, allowing for visualization of necrotic myocardial tissue.5 Later, Werner et al. studied the clinical applications of 99mTc-PYP and demonstrated its high sensitivity (~90%) in transmural myocardial infarction but less so in subendocardial infarcts (~38%).6 This was a novel discovery, as it allowed and set the stage for noninvasive cardiac imaging of infarcted myocardium, complementing electrocardiograms and serum enzymes creatine phosphokinase myocardial specific.
While it remained a major tool for AMI detection in the 1970s and 1980s, its use gradually declined in the 1990s due to the use of better diagnostic tools and treatments, including cardiac-specific biomarkers such as troponins, the development of echocardiography, percutaneous coronary intervention, and thrombolytic therapy.7 - 9
Early Recognition of Cardiac Amyloidosis (1980s)
In the early 1980s, 99mTc-PYP uptake was unexpectedly observed in many patients with cardiac amyloidosis. Wizenberg et al. reported diffuse, intense myocardial uptake in 10 consecutive patients with biopsy-proven amyloidosis, all exhibiting restrictive cardiomyopathy.10 Other studies during the 1980s suggested the usefulness of 99mTc-PYP in the diagnosis of cardiac amyloidosis; however, due to variable diagnostic accuracy in undifferentiated ATTR and AL subtypes, its clinical use was limited.11 Clinical interest also remained limited due to a lack of disease-modifying therapies and some concerns over nonspecific tracer accumulation in the heart and other tissues.
Resurgence for ATTR Cardiac Amyloidosis and Technical Refinements (2000s-Present)
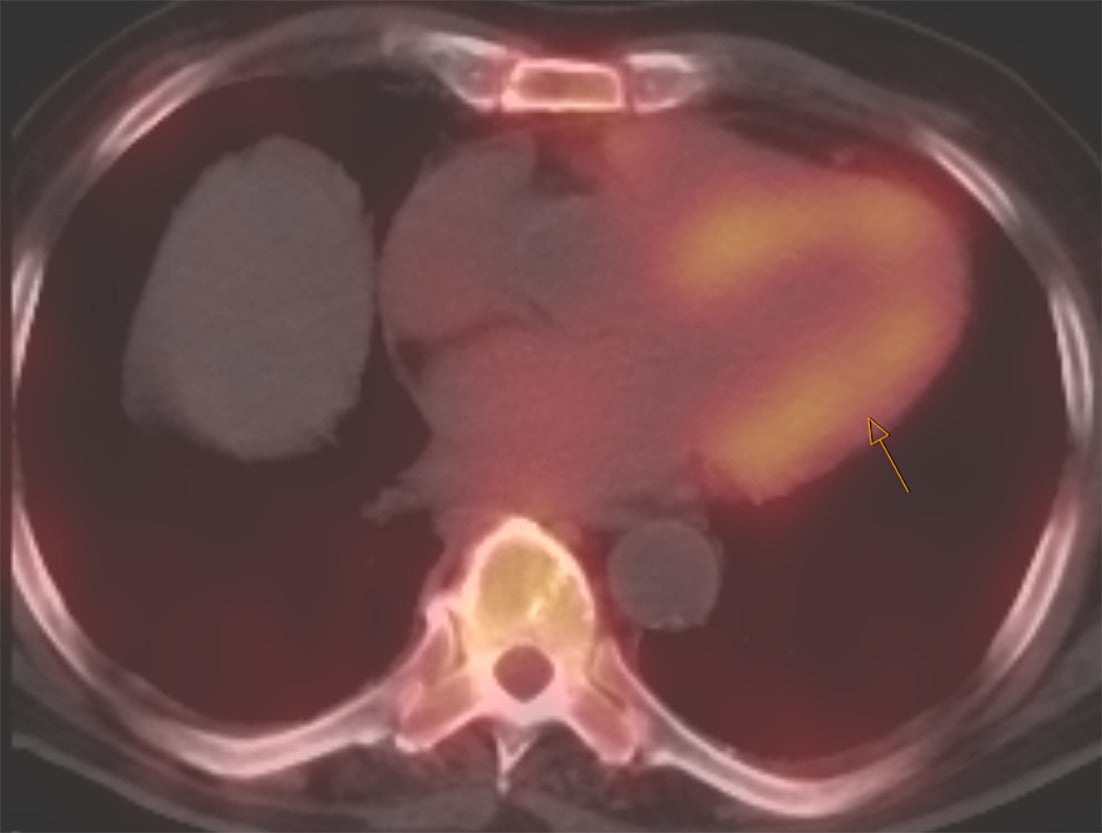
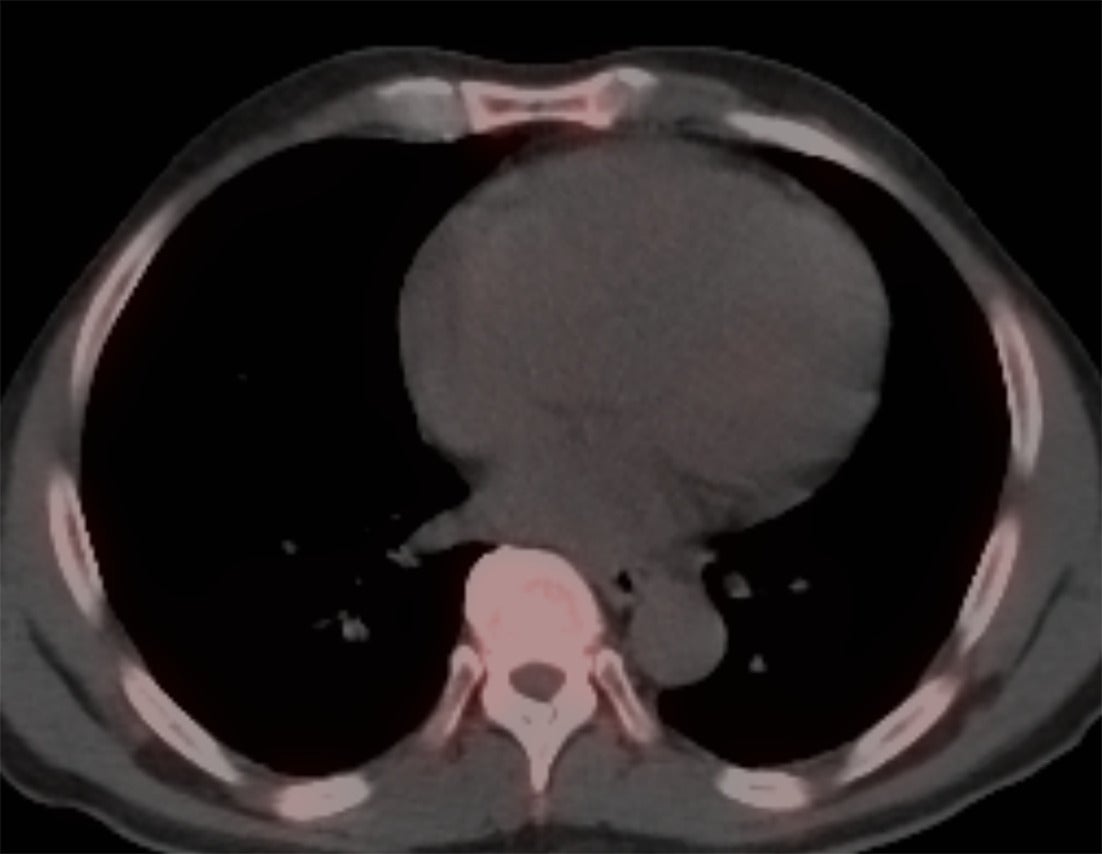
Perugini et al. in 2005 demonstrated that 99mTc-DPD (diphosphono-1,2-propanodicarboxylic acid) scintigraphy could noninvasively differentiate ATTR cardiac amyloidosis from AL amyloidosis with high accuracy.12 ATTR cardiac amyloidosis showed uptake for bone radiotracers, whereas AL cardiac amyloidosis showed minimal to no uptake ( Figures 1 , 2 ). They also developed the visual Perugini grading scale (0‐3) for semiquantitative assessment as below.12
SPECT/CT image of the chest with strong myocardial uptake (shown by arrow) consistent with TTR amyloidosis.
SPECT/CT image of the chest without any myocardial uptake, ruling out the possibility of TTR amyloidosis.
Perugini grading scale:
-
Grade 0: no cardiac uptake and normal rib uptake.
-
Grade 1: Cardiac uptake, which is less than rib uptake.
-
Grade 2: Cardiac uptake with intensity, similar to rib uptake.
-
Grade 3: Cardiac uptake greater than rib uptake with mild/absent rib uptake.
Three clinically available bone-avid radiotracers—99mTechnetium (Tc)-bisphosphonate derivatives (99mTc-pyrophosphate), 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD), and 99mTc hydroxymethylene-diphosphonate (99mTc-HMDP)—are used for the detection of myocardial ATTR amyloid deposition, but not AL amyloid.13 99mTc-PYP is primarily used in the United States, while 99mTc-DPD and 99mTc-HMDP are predominant in Europe. However, these tracers are viewed as interchangeable and can be used as alternatives to each other because of similar diagnostic performance for ATTR amyloidosis.14 - 16
Continued advancements in nuclear imaging, particularly in SPECT/CT, led to improved anatomical localization and reduced blood-pool artifact, and eventually better diagnostic ability. In 2013, Bokhari et al. developed a standardized method, the heart-to-contralateral (H/CL) ratio, for differentiating AL versus ATTR amyloidosis. 45 patients underwent 99mTc-PYP planar and SPECT imaging, and cardiac uptake was assessed with both a semiquantitative visual score (0 with no uptake to three with diffuse uptake) and by a quantitative method by drawing a region of interest over the heart and contralateral lung (H/CL ratio). They demonstrated that an H/CL ratio >1.5 had 97% sensitivity and 100% specificity for ATTR.17 They concluded that Tc-99m PYP can be a simple, widely adopted method for diagnosing ATTR amyloidosis.
Maurer et al., in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT), found that patients on the drug tafamidis, a TTR stabilizer, had reduced mortality and cardiovascular-related hospitalizations and improved functional capacity compared to those on placebo.18 Its approval by the FDA in 2019 for ATTR amyloidosis led to widespread adoption of 99mTc-PYP imaging in the ATTR diagnostic algorithm. As of 2025, the FDA has approved two more drugs for ATTR: acoramidis (2024), another TTR stabilizer, and vultrisiran (2025), a TTR silencer.18 Acoramidis prevents amyloid formation by stabilizing the TTR tetramer, while vultrisiran prevents amyloid formation through degradation of TTR messenger RNA.18
Current Guidelines and Protocols
Cardiac amyloidosis should be suspected in individuals with heart failure and thickened ventricles ( >1.2 cm) with relative apical sparing and grade two or greater diastolic dysfunction on echocardiography.1 However, due to the lack of specificity of echocardiography, cardiac magnetic resonance imaging (CMR) aids in identifying the diffuse, subendocardial, and transmural late-gadolinium enhancement (LGE) pattern seen in patients with cardiac amyloidosis.
Subendocardial LGE is more commonly seen in AL, and transmural LGE in ATTR cardiac amyloidosis.19, 20 Moreover, both native (non-contrast) and post-contrast T1 mapping for calculating expanded extracellular volume (ECV) show excellent diagnostic ability for cardiac amyloidosis. Baggiano et al. in their study found that a native T1 < 1036 ms yields a negative predictive value of 98%, and a native T1 > 1164 ms yields a positive predictive value (PPV) of 98% for cardiac amyloidosis, with an ECV cutoff of 0.37 as an excellent diagnostic tool within the range.21
Multi-society expert consensus 2021 suggests blood and urine exam for monoclonal proteins and 99mTc-PYP cardiac scintigraphy as key investigations for suspected cardiac amyloidosis, clinically or based on echocardiogram and CMR.1 If 99mTc-PYP is negative with no monoclonal proteins in the blood and urine, then cardiac amyloidosis is very unlikely. Grade 2- or 3-positive 99mTc-PYP scintigraphy without evidence of monoclonal proteins in blood and urine is diagnostic of ATTR cardiac amyloidosis (specificity and PPV > 98%).22
When monoclonal proteins are detected in both blood and urine, a histological diagnosis should be confirmed by endomyocardial biopsy. This is necessary because low-grade 99mTc-PYP uptake lacks specificity for ATTR cardiac amyloidosis, and approximately 20% of patients with AL amyloidosis may also show low to moderate 99mTc-PYP uptake.16, 22 Therefore, ruling out AL amyloidosis—by confirming normal serum and urine light chain profiles—is essential for an accurate diagnosis of ATTR amyloidosis.23 Serum protein electrophoresis alone is insensitive and unreliable for detecting AL amyloidosis. Instead, serum and urine immunofixation, along with serum-free light chain assays, are critical diagnostic tools.1 For confirmed ATTR amyloidosis, TTR gene sequencing is required to differentiate between ATTRwt and variant (ATTRv) forms.1
The standard imaging protocol involves an intravenous injection of approximately 15‐20 mCi (555‐740 MBq) of 99mTc-PYP. Imaging is performed at early (1 h) and delayed (2‐3 h) phases using planar gamma cameras and SPECT/CT.1, 23 Relying solely on planar imaging may lead to indeterminate findings; therefore, SPECT/CT acquisition at 2‐3 h is essential, as it allows for improved anatomical localization of radiotracer uptake and better visualization of early cardiac amyloid deposition sites, including atrial and septal regions.16 Both planar and SPECT images should be evaluated using semi-quantitative visual scoring (Perugini grading). Notably, recent addenda to the multisociety expert consensus recommendations have removed the use of quantitative H/CL ratio criteria due to inconsistent results, emphasizing instead visual assessment and SPECT interpretation.16
Conclusion
Since its introduction in the 1970s as a tool for early myocardial, 99mTc-PYP scintigraphy has become a cornerstone diagnostic modality for ATTR). As cardiac amyloidosis is increasingly recognized as a cause of heart failure among elderly patients, 99mTc-PYP imaging plays a central role in the diagnosis of ATTR cardiac amyloidosis after the exclusion of AL amyloidosis in the absence of monoclonal proteins in the blood and urine. A distinct advantage of 99mTc-PYP imaging is its wide accessibility and very high specificity for the diagnosis of ATTR amyloidosis noninvasively without the need for endomyocardial biopsy.
References
Citation
Jamal F, MBBS J, MBBS SK, Chaudhary SR.99mTc-Pyrophosphate Scintigraphy: History, Evolution, and Role in Cardiac Amyloidosis. Appl Radiol. 2025; (1):1 - 4.
doi:10.37549/AR-D-25-0090
November 1, 2025