Thymic carcinoma with pulmonary metastasis
Images

Thymic carcinoma with pulmonary metastasis
Findings
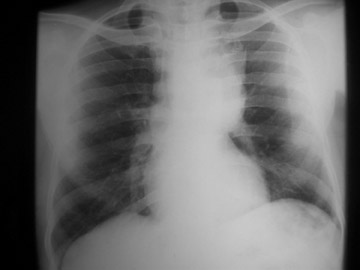
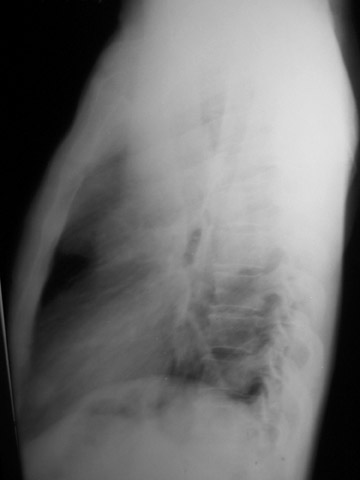
The chest radiograph shows a large left mediastinal soft tissue mass with smoothly marginated borders and no calcification (Figure 1A). A rounded soft tissue opacity was seen in the retrosternal clear space on the lateral projection (Figure 1B).
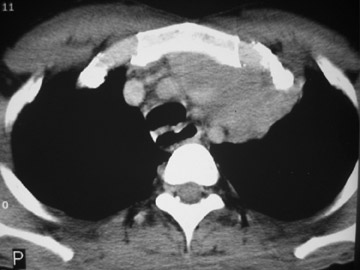
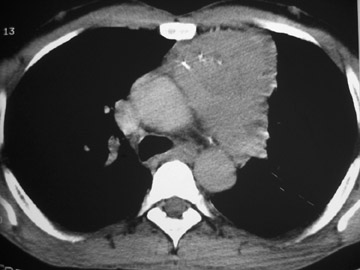
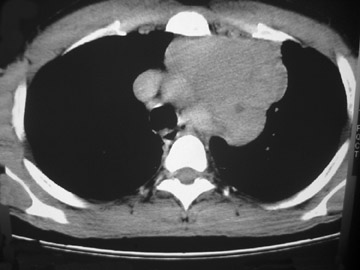
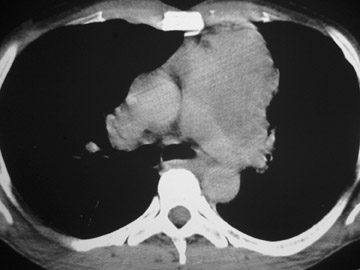
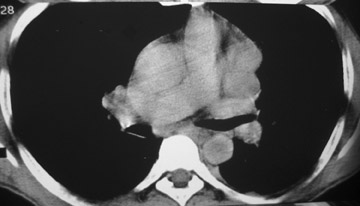
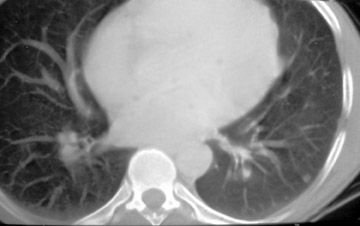
The CT scan showed a lobulated left anterior mediastinal soft tissue mass with eccentrically placed clumps of calcification (Figure 2B). The tumor shows moderate heterogeneous enhancement following contrast administration with few areas of decreased attenuation—likely corresponding to cystic changes or foci of hemorrhage and necrosis. The mass shows obliteration of mediastinal fat (Figure 2C), and the absence of fat planes between the mass and mediastinal structures is also evident, suggesting invasion. Postcontrast scans taken at the level of the arch of the aorta and main pulmonary artery show focal loss of planes with the great vessels. There are also enlarged lymph node(s) in the aorto-pulmonary window region (Figure 3) and small metastatic lesions in the left lung (Figure 4). In view of the clinico-radiological findings, a diagnosis of malignant anterior mediastinal mass was suggested—likely invasive thymoma or thymic carcinoma. Ultrasound-guided fine-needle aspiration and core biopsy confirmed a diagnosis of thymic carcinoma.
The patient was examined again to rule out possible associated immunological disorders, such as myasthenia gravis, red cell aplasia, etc. There was no history of muscle weakness, difficulty swallowing, or ptosis, and further immunological investigations, electromyography, and nerve conduction study results were within normal limits. The patient was treated with an intravenous (IV) chemotherapeutic regimen consisting of cisplatin, doxorubicin, and cyclophosphamide, with amifostine.
Discussion
Thymoma is the most common tumor of the anterior mediastinum and the most common primary tumor of the thymus. Since thymomas are rare in children and young adults, 70% of them are found in adults in the fifth and sixth decades of life. There is an approximately equal male-to-female ratio. The majority of thymomas are found in the anterior mediastinum. Other locations may be the neck and posterior mediastinum.
Approximately 20% to 50% of thymomas are asymptomatic, and the frequency of thoracic symptoms is related to compression or invasion of adjacent mediastinal structures. Compression of the trachea, recurrent laryngeal nerve, or esophagus may produce cough, dyspnea, chest pain, respiratory infection, hoarseness, or dysphagia. Invasion of the adjacent cardiovascular structures may produce superior vena caval syndrome. Thymomas can also be associated with autoimmune and paraneoplastic syndromes, such as pure red cell aplasia (50% of patients with red cell aplasia will have thymoma, and 5% of patients with thymoma will have red cell aplasia), hypogammaglobulinemia (10% of patients with thymoma will have hypogammaglobulinemia, and 5% of patients with hypogammaglobulinemia will have thymoma), endocrine disorders, cutaneous disorders, and connective tissue disorders.1 Evidence linking thymoma with other second malignancies (of the rectum, thyroid, breast, and lung) has been suggested by clinical studies. Distant metastases are uncommon at initial presentation. However, when present, the most common metastatic site at presentation is the pleura.
CT is the modality of choice because it is more sensitive than chest radiography for the detection of small thymomas and in revealing tumor infiltration of the surrounding mediastinal structures and adjacent lung. Furthermore, CT is an excellent modality for the evaluation of pleural and extrapleural seeding by the tumor. Calcification, even if subtle, can be easily detected with CT. Magnetic resonance imaging is an excellent noninvasive method of evaluating possible vascular involvement by thymoma without the need for IV contrast medium. Thymomas are isointense relative to skeletal muscle on T1-weighted images and have increased signal intensity (approaching that of fat) on T2-weighted images. Cystic areas are seen as low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.
The differential diagnosis of solid invasive anterior mediastinal masses includes lymphoma (ill-defined mass with associated regional lymphadenopathy), bronchogenic carcinoma, neuroendocrine tumors, benign germ cell tumors (sharply defined masses with cystic areas and mixed areas of calcification and fat), and nonseminomatous germ cell tumors (large, poorly defined masses with zones of hemorrhage and necrosis).
The histologic classification of thymic epithelial tumors and its relationship with prognosis still remains controversial. Several classifications, based on different pathologic criteria, have been proposed in the past. Bernatz et al2 classified thymomas on the basis of the ratio of lymphocytes to epithelial cells and the shape of epithelial cells into 4 subtypes: predominantly spindle cell; predominantly lymphocytic; predominantly mixed; and predominantly epithelial.2 Müller-Hermelink et al3 classified thymomas into 6 categories based on resemblance of tumor to cortex or medulla of normal thymus (from well-differentiated to poorly differentiated tumors): medullary thymomas; mixed thymomas; predominantly cortical (organoid) thymomas; cortical thymomas; well-differentiated thymic carcinomas; and high-grade thymic carcinomas.3 This system has prognostic significance, independent of tumor stage. The World Health Organization (WHO) established a new system based on the morphology of the epithelial cells as well as the lymphocyte-to-epithelial cell ratio. Six categories of thymomas divided into 2 subtypes were defined: unique thymic tumors (types A, AB, B1, B2, and B3); and malignant thymomas (type C). The relationship of the WHO classification and previous histologic classifications is as follows: type A corresponds to spindle cell type or medullary type thymoma; AB corresponds to mixed type; B1 corresponds to lymphocyte-rich type, lymphocytic type, predominantly cortical type, or organoid type; B2 corresponds to cortical type; B3 corresponds to epithelial type, squamoid type, or atypical thymoma, or well-differentiated thymic carcinoma; and type C corresponds to thymic carcinoma. This classification tries to reflect both the clinical and the functional features of thymic epithelial tumors.4 Although CT is of limited value in differentiating histologic subtypes according to the WHO classification, CT findings may serve as predictors of postoperative recurrence or metastasis for thymic epithelial tumors.5 Low-risk thymomas include subtypes A, AB, B1; high-risk thymomas consist of types B2 and B3. The presence of smooth contours and a round shape are most suggestive of a type A tumor, while a lobulated, irregular contour is more often seen in high-risk thymomas. The combination of homogeneous enhancement and a high degree of enhancement is suggestive of type A and AB tumors. CT is of limited value, however, in differentiating type AB, B1, B2, and B3 tumors. Mediastinal lymphadenopathy, great vessel invasion, and mediastinal fat infiltration are most suggestive of type C tumor or thymic carcinomas. Tumors with a lobulated or irregular contour, an oval shape, mediastinal fat, or great vessel invasion, and pleural seeding show significantly more frequent recurrence and metastasis. Type A, AB, or B1 thymomas have a less aggressive nature than type B2 or B3 thymomas and, therefore, have more chances of complete resection.
Recently, it has been suggested that it may be possible to differentiate between atypical thymoma and thymic carcinoma based on CT findings. Thymic carcinomas are larger tumors, and the findings of invasion of the great vessels, lymphadenopathy, phrenic nerve palsy, and lung or distant metastases are seen only in patients with thymic carcinoma. The tumors occur in a broad age range of patients (average age 46 years), have a low incidence of paraneoplastic syndromes, and have a poor prognosis.6
Although tumor histology may influence overall prognosis, tumor stage is a more important overall survival indicator, as confirmed by a number of published studies. Staging of thymoma is based on the presence or absence of an intact tumor capsule. The staging system proposed by Masaoka et al7 is postsurgical and has been widely adopted. Stage I thymomas do not show capsular invasion. Stage II lesions show microscopic invasion of the capsule, mediastinal fat, or surrounding pleura. Stage III tumors invade surrounding organs and structures such as the lung, pericardium, superior vena cava, and aorta. Stage IVa involves pleural or pericardial dissemination. Stage IVb involves lymphogenous or hematogenous metastases. For the majority of patients who present with a localized tumor, surgical extirpation by median sternotomy remains the standard of choice. However, since thymomas may have either pleural or pericardial implants, both pleural cavities should be widely opened and explored at surgery. Adjuvant radiotherapy seems to improve local control and survival. In more advanced disease, systemic therapy has been shown to produce a 50% to 80% objective response rate. These observations have led to the development of multimodality therapy for the treatment of patients with advanced thymoma.
Conclusion
We have discussed the various histological classifications for thymomas as well as the widely accepted postsurgical Masaoka staging, which is an independent predictor of outcome and survival. Though CT cannot be used to distinguish between various histological subtypes, it can be used to identify thymomas as low risk or high risk. Also, when a large thymic tumor is associated with the invasion of great vessels, lymph node enlargement, phrenic nerve palsy, or extrathymic metastases on CT, thymic carcinoma rather than atypical thymoma should be considered, as in our case.
- Souadjian JV, Enriquez P, Silverstein MN, Pépin JM. The spectrum of diseases associated with thymoma. Coincidence or syndrome? Arch Intern Med. 1974;134:374-379.
- Bernatz PE, Harrison EG, Clagett OT. Thymoma: A clinicopathologic study. J Thorac Cardiovasc Surg. 1961;42:424-444.
- Müller-Hermelink HK, Marx A. Thymoma.Curr Opin Oncol.2000;12:426-433.
- Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncological behavior of thymoma: A clinical study of 273 patients. Cancer. 2002; 94:624 -632.
- Jeong YJ, Lee KS, Kim J, et al. Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis? AJR Am J Roentgenol. 2004;183:283-289.
- Jung KJ, Lee KS, Han J, et al. Malignant thymic epithelial tumors: CT-pathologic correlation. AJR Am J Roentgenol. 2001;176:433-439.
- Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485-2492.