FDA Fast Tracks [18F]PI-2620 from Life Molecular Imaging for Some Tau PET Scans
Images
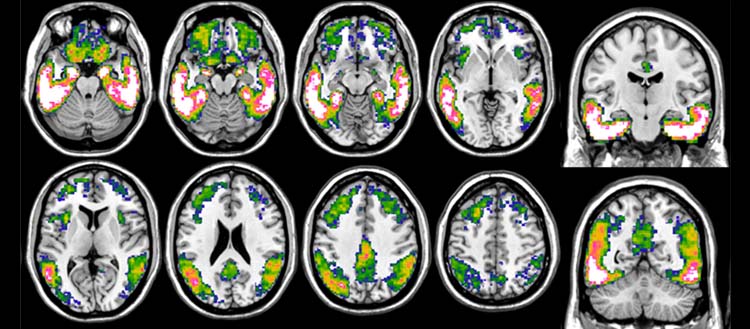
Life Molecular Imaging (LMI) announced that the US FDA granted Fast Track Designation to [18F]PI-2620 Injection, an investigational PET imaging agent targeting tau neurofibrillary tangles. The Fast Track Designation has been granted for clinical development in Alzheimer's disease (AD), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD).
The FDA's Fast Track program is designed to accelerate the development and review of drugs that address serious conditions and fulfill unmet medical needs. This designation underscores the significant potential of [18F]PI-2620 to improve diagnosis of these three devastating neurodegenerative diseases.
[18F]PI-2620 is a next-generation, F18-labeled PET imaging agent currently in Phase 3 clinical development for detecting tau pathology in Alzheimer's disease. The compound is also being investigated in other neurodegenerative diseases and settings by many academic researchers and in drug development trials. Tau proteins are a hallmark of several neurodegenerative disorders including AD, PSP, CBD, and frontotemporal lobar dementia (FTLD). The ability to accurately image tau pathology could significantly enhance disease diagnosis and patient care.
"Receiving Fast Track Designation from the FDA is a major milestone that highlights the promise of [18F]PI-2620 in addressing the critical need for effective diagnostic tools in Alzheimer's disease, progressive supranuclear palsy, and corticobasal degeneration," said Andrew Stephens, Chief Medical Offcer at LMI. "This designation not only validates our approach but also facilitates closer collaboration with the FDA to expedite the development of [18F]PI-2620. We are committed to advancing this important imaging agent with the potential to make a meaningful difference for patients who need accurate and accessible Tau PET imaging."
Related Articles
Citation
FDA Fast Tracks [18F]PI-2620 from Life Molecular Imaging for Some Tau PET Scans. Appl Radiol.
September 4, 2024