Aneurysms—All you need to know
Images

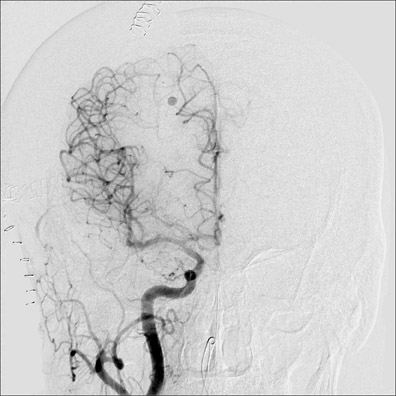
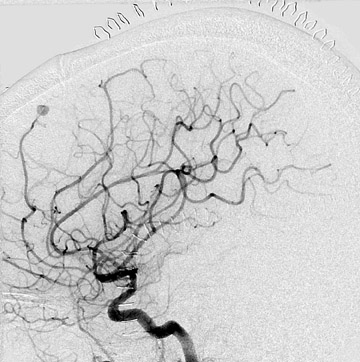
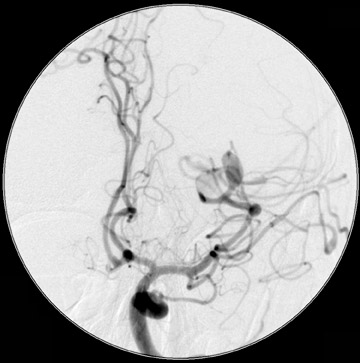

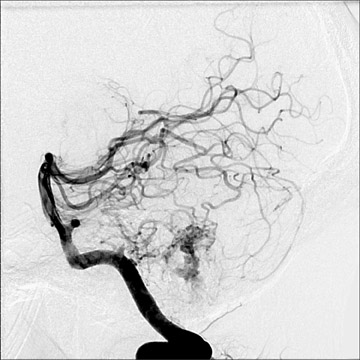




Dr. Bagley is an Associate Professor of Radiology and Neurosurgery, University of Pennsylvania School of Medicine, Philadelphia, PA.
Aneurysms and their risks, diagnosis, and management are controversial topics at the beginning of the 21st century. This article reviews the epidemiology of aneurysms, the roles of computed tomography (CT), CT angiography (CTA), magnetic resonance imaging (MRI), MR angiography (MRA), and conventional angiography in the imaging of aneurysms, and current surgical and endovascular therapy.
Epidemiology
Aneurysms are estimated to occur in approximately 2% to 6% of the population (based upon autopsy and angiographic studies). 1-5 They occur more commonly in women and more frequently in association with polycystic kidney disease, connective tissue disorders (including Marfan's syndrome and Ehrles-Danlos syndrome), moya-moya syndrome, aortic coarctation, Takayasu's arteritis, neurofilar dysplasia (FMD). 6-9 There is also an increased incidence of aneurysms in patients with ≥2 first-degree relatives who have had aneurysms. In patients with such a positive family history, aneurysms are often multiple and relatively larger, are more often located along the middle cerebral artery, and have a tendency to rupture earlier in life. 10,11
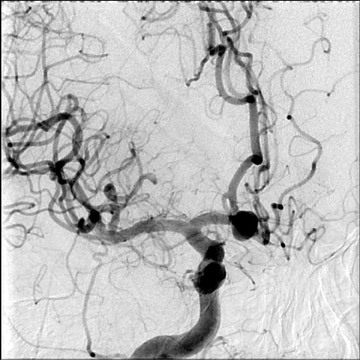
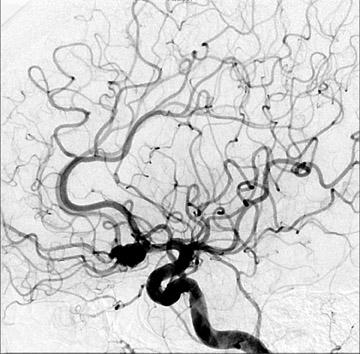
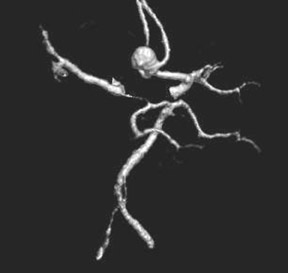
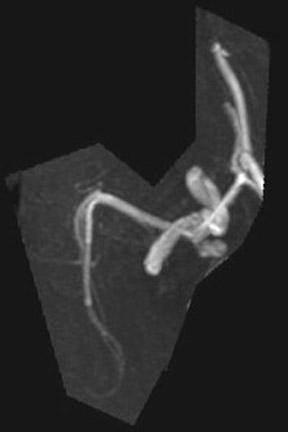
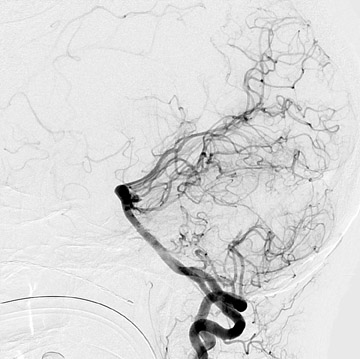
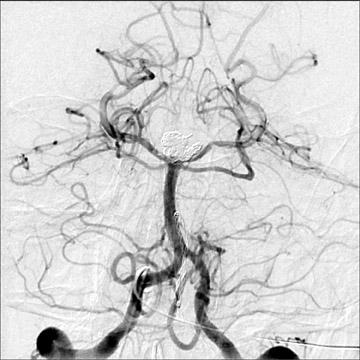
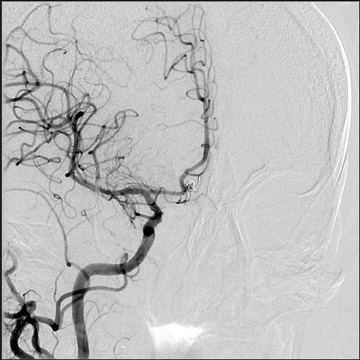
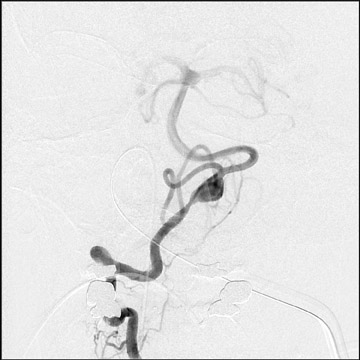
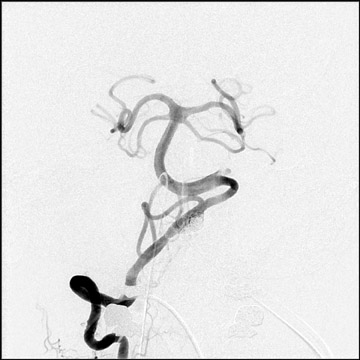
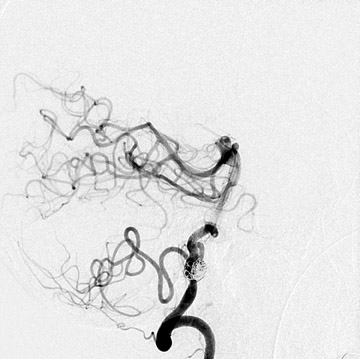
Congenital developmental aneurysms may be saccular or fusiform. Saccular aneurysms typically arise at vascular branch points, often arise from congenital areas of weakness in the arterial wall and enlarge over time, and usually do not present until adulthood 12 (Figure 1). Fusiform aneurysms more frequently occur in older patients, are generally secondary to atherosclerosis, and may develop intraluminal thrombus 9 (Figure 2). Cigarette smoking and, to a lesser extent, hypertension (as well as female gender) likely contribute to formation and growth of both saccular and fusiform aneurysms. 13-16
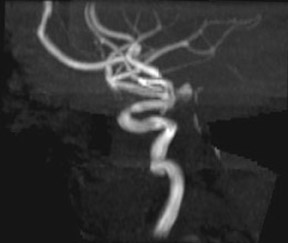
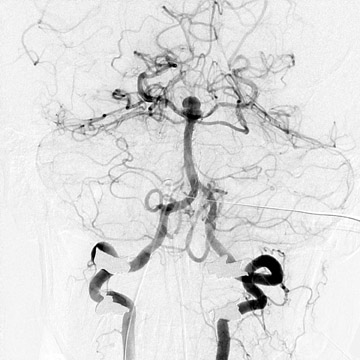
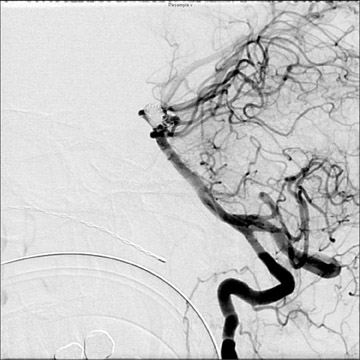
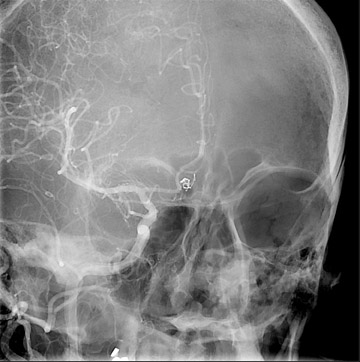
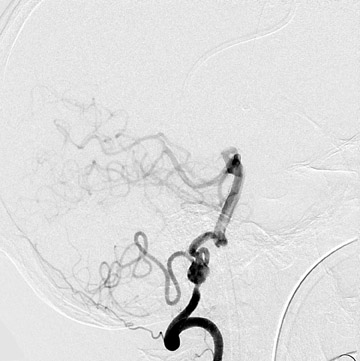
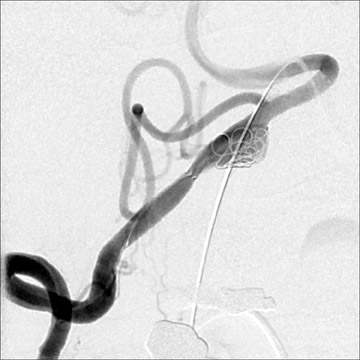
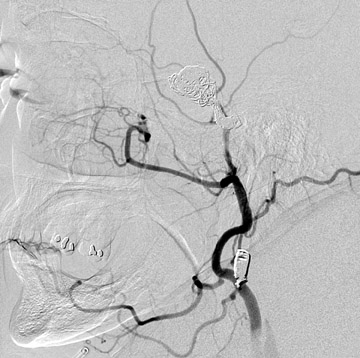
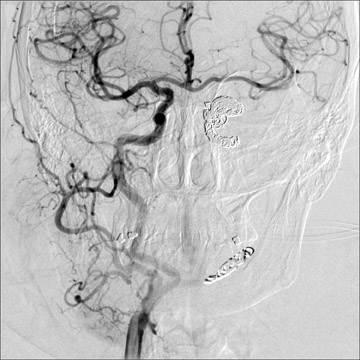
Traumatic, infectious, and neoplastic aneurysms are less common, together accounting for <10% of intracranial aneurysms. Traumatic aneurysms (approximately 1% of intracranial aneurysms) are often distal and most often result from penetrating trauma, adjacent fractures, or impact of the vessel with the falx cerebri or tentorium cerebelli (Figure 3). 6,9,17 Dissecting aneurysms contain a component of the native vessel wall but result from intimal disruption. 12 They are more likely to occur in patients with FMD, connective tissue disorders, polyarteritis nodosa, and syphilis. 8,9,12,18 Infectious and neoplastic aneurysms result from direct invasion and destruction of the arterial wall. Infectious aneurysms may be seen in the setting of endocarditis, meningitis, and thrombophlebitis. Typical causative organisms are Streptococcus viridans, Aspergillus fumigatus, and Aspergillus flavus (Figure 4). 6,9,12,18-20 Neoplastic aneurysms are most often seen with atrial myxomas and metastatic choriocarcinoma. 6,9,12
Serpentine aneurysms are rare partially thrombosed giant aneurysms, with separate inflow and outflow channels. The residual lumen of a serpentine aneurysm is made up of channels formed within the thrombus, not the true lumen of the parent vessel. Flow within these channels is typically sluggish, and the outflow channel supplies normal cerebral vasculature. More than 50% of such aneurysms arise from the middle cerebral artery. Repeated localized hemorrhage, formation of organized thrombus, and calcification are characteristic of these aneurysms. On cross-sectional imaging studies, these aneurysms may mimic neoplasms, appearing as heterogeneous, partially enhancing masses. Also, symptoms often mimic those of neoplasms because of local mass effects (producing sensory and motor deficits and/or seizures) and are occasionally secondary to intracranial hemorrhage. 12,21-24
Aneurysms may also be seen in association with arteriovenous malformations (Figure 5). When superselective angiography has been performed, their incidence has been reported to exceed 50%. These aneurysms are typically flow-related, may be present along feeding pedicles or within the nidus, and may enlarge or regress with alterations in the hemodynamics of the arteriovenous malformation. 25,26
Approximately 15% to 45% of aneurysms are multiple, and their most common locations are along the anterior communicating artery complex, along the supraclinoid internal carotid artery, at the middle cerebral artery bifurcation, at the basilar artery tip, and at the origin of the posterior inferior cerebellar artery. 27
Risk of aneurysmal subarachnoid hemorrhage
Many studies published prior to the 1990s suggested that aneurysms carried an approximately 1% to 2% chance of rupture per year. 28-30 This belief has since been challenged by multiple studies of unruptured aneurysms, most notably, the International Study of Unruptured Intracranial Aneurysms (ISUIA), which published data on a large cohort in the New England Journal of Medicine in 1998 31 and in The Lancet in 2003. 32 Among 4060 patients with unruptured intracranial aneurysms, 1692 were observed with a total of 6544 patient years of follow-up. The studies concluded that the risk of aneurysm rupture was dependent on aneurysm size, location, and prior history of subarachnoid hemorrhage (SAH). 31,32 In the more recent study, aneurysms were categorized by the following locations-cavernous internal carotid artery, anterior circulation, and posterior circulation (including the posterior communicating artery origin) and by the following sizes: <7 mm, 7 to 12 mm, 13 to 24 mm, and >24 mm. Patients with a prior history of SAH were determined to be at greater risk of incurring another SAH, but risk did not correlate with aneurysm size. 31,32 In this study, in patients without a prior history of SAH, no observed aneurysms measuring <7 mm in the anterior circulation ruptured. Posterior circulation aneurysms were associated with a higher risk of rupture than were anterior circulation aneurysms, and cavernous internal carotid artery aneurysms, as expected, were associated with minimal risk. However, giant aneurysms, particularly those in the posterior circulation, were associated with high risk of rupture of up to 10% per year. 31,32
While ISUIA has provided valuable information on the natural history of intracranial aneurysms and has certainly influenced the management of these lesions, this study has multiple limitations. First, there is a selection bias in this study. More than half of the patients presenting received surgical or endovascular therapy for their unruptured aneurysms. In general, patients with aneurysms considered at low risk of rupture and patients judged to be at high risk for intervention by the evaluating clinician were followed. The inclusion of cavernous internal carotid artery aneurysms in this study is also controversial. Truly cavernous aneurysms are not within the subarachnoid space, though it is possible for larger aneurysms to thin and breach the integrity of the dura, thus allowing extension into the subarachnoid space. Even so, no subarachnoid hemorrhages were observed secondary to cavernous internal carotid artery aneurysms that measured <13 mm. The inclusion of posterior communicating artery origin aneurysms with posterior circulation aneurysms also conflicts with earlier studies.
Imaging of aneurysms
Catheter angiography
While the risk of aneurysmal SAH is a controversial topic, strategies for the diagnosis and management of aneurysms are even more controversial.
Catheter angiography (using multiple projections, high-resolution subtracted images, and rapid filming techniques) has long been the gold standard for aneurysm diagnosis. More recently, rotational angiography has provided 3-dimensional (3D) data and is increasingly becoming part of the standard evaluation of the intracranial vasculature. However, catheter angiography has associated risks, including those of allergic contrast reaction, nephrotoxicity, hemorrhage, infection, and vascular injuries, including dissections, occlusions, and arteriovenous fistulae. The risk of transient neurologic deficits has been reported to be 1% to 3%, with a risk of permanent deficits of 33-38 Risk increases with advanced patient age, the presence of atherosclerosis, and other concurrent diseases. Risk of the procedure is inversely proportional to the experience of the angiographer.
CT angiography
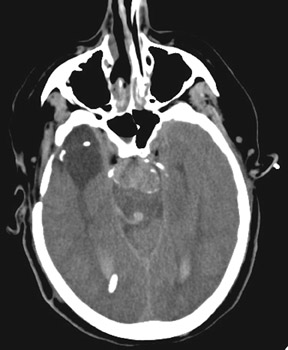
Increasingly, CTA has been used for the evaluation of aneurysms and of patients with intracranial hemorrhage (Figure 6). CT angiography is performed on multidetector CT scanners, during the dynamic administration of approximately 80 to 100 mL of contrast (at approximately 4 mL/second) and is typically reconstructed at 0.5 to 0.7 mm intervals in the axial plane. Two-dimensional (2D) and 3D reformatted images are then created, often utilizing maximum-intensity projection (MIP) and volume-rendering (VR) techniques. Many studies have reported sensitivities of up to and exceeding 95% for CTA and MRA in the detection of aneurysms >3 mm in size. 39-41 Like MRA, the sensitivity of CTA for the detection of aneurysms <2 mm in size is poor. CT angiography may certainly play a role in aneurysm screening, particularly in patients with contraindications to MRA, though the utility and cost-effectiveness of screening are also controversial topics. CT angiography provides valuable information about complex aneurysms and often complements conventional 2D angiography by better defining the relationship of the aneurysm to the parent and branch vessels, by delineating its relationship to the skull base and by demonstrating thrombosed portions of the aneurysm.
CT angiography has become the initial vascular imaging modality of choice for SAH in multiple institutions. 42-44 Clinicians at Massachusetts General Hospital conducted a prospective trial of CTA as the sole pretreatment imaging modality for aneurysms. In this study, 223 patients were evaluated, and treatment (surgical or endovascular) was initiated based on only CTA in 82% of patients. 43 When patients require urgent hematoma evacuation, CTA is most time-efficient and is the imaging modality of choice.
CT angiography also has a role-though, again, a controversial one-in the evaluation in perimesencephalic hemorrhage (Figure 45,46 Rinkel et al 45 have suggested that CTA is cost-effective as the sole imaging modality in perimesencephalic hemorrhage, which is most often nonaneurysmal and possibly secondary to venous or capillary hemorrhage. In this entity, blood is seen only anterior to the brainstem, in the ambient cisterns, and in the basal sylvian fissures without lateral sylvian fissure or interhemispheric blood. Hemorrhage typically rapidly resolves, and patients most often have an uncomplicated course without development of vasospasm or rebleeding. Angiographic imaging is, however, required to exclude a basilar tip aneurysm (approximately 9% of which present with a similar hemorrhage pattern). 47 Rinkel 45 and Kershenovich 46 have argued that CTA is sufficient to do this.
There are, however, limitations of CTA, including decreased sensitivity for detection of aneurysms measuring <3 mm, relatively poor demonstration of perforators, and decreased sensitivity for detection of arteriovenous malformations, dural arteriovenous fistulas, and vasculitis. Additionally, sensitivity of CTA is dependent on the training of the interpreter, and upon the postprocessing techniques employed. 39,40,42
MR angiography
As above, MRA, typically employing the 3D time-of-flght technique, performed at high field strength (≥1.5T) with image segmentation, is reported to be approximately 95% sensitive for detecting aneurysms measuring at least 3 mm (Figure 8). 41 It does not require the administration of contrast material or the use of ionizing radiation, and, as such, can play a role in aneurysm screening and aneurysm imaging in patients with contraindications to contrast administration. MR angiographic images are, however, subject to degradation by multiple artifacts, and visualization of the distal vasculature and small perforators is suboptimal and inferior to CTA. 41,48 Despite these current limitations, with increased usage of higher field strength systems (3T and 7T), the role of MRA in aneurysm evaluation is likely to increase as image resolution improves and acquisition times decrease.
Aneurysm therapy
Surgical
Treatment strategies for aneurysms remain very controversial. Surgical therapy continues to be used widely, although multiple recent studies have challenged previously held beliefs about its associated risks and efficacy. Postoperative angiograms have been reported to reveal unexpected vascular occlusions in up to 12% of cases and incomplete aneurysm obliteration in 4% to 18% of cases in various series. 49 Intraoperative angiography may be used to allow more timely clip repositioning, and the results of intraoperative angiography have been reported to alter surgical management in 15% to 30% of cases. 50-56 This technique has been shown to be clearly cost-effective in cases of ruptured intracranial aneurysms. 55 While some authors advocate its use only in select elective cases, Klopfenstein et al 56 have shown that surgeons are generally unable to predict its utility.
Meta-analysis of earlier studies suggested that elective aneurysm surgery was accompanied by a morbidity of approximately 4% and mortality of approximately 1%. 57 Based upon these assumptions and the possibly flawed assumption that aneurysms rupture at a rate of approximately 1% to 2% per year, King et al 58 concluded that microsurgical repair of an unruptured aneurysm was cost-effective if the patient had a life expectancy of at least 13 years and if the patient's quality of life suffered because of the negative psychological impact of knowledge of the aneurysm. Data obtained from the ISUIA, however, suggests that the complication rate of elective aneurysm surgery, particularly in older patients, is much higher than had been previously reported. In the ISUIA study, the 1-year morbidity and mortality for elective procedures was 12.2% for surgical repair and 9.5% for endovascular therapy (not controlled for comorbidities). Surgical complication rates correlated with patient age, with a significant increase in morbidity and mortality in patients older than 50 years and an even more dramatic increase in patients older than 60 years. In fact, in patients older than 64 years, surgical complication rates exceeded 30%. 32 Based on this data concerning aneurysm rupture rates and expected morbidity and mortality of treatment, some authors have concluded that there is no benefit for therapy of unruptured anterior circulation aneurysms <7 mm or in patients with life expectancy <15 to 35 years. 59
Endovascular
Aneurysm therapy has significantly evolved since the introduction of detachable embolization coils in the 1990s (Figure 9). Endovascular therapy continues to evolve with the introduction of multiple new coils, intracranial stents, and bioactive materials. As such, the risks and efficacy of endovascular treatments have continued to change. During the last 2 decades, the number of aneurysms treated with endovascular techniques has dramatically increased. Approximately 80% to 85% of aneurysms are amenable to coil embolization, but when first introduced, endovascular therapy was often reserved for aneurysms in surgically unfavorable locations or of unfavorable configurations and for patients in poor clinical condition, who were deemed to be poor surgical risks. 60-64 These factors produced significant bias when attempting to compare outcomes of endovascular and surgical therapy. The international subarachnoid aneurysm trial (ISAT) published in Lancet in 2002 60 and 2005 61 compared the outcomes of patients with ruptured intracranial aneurysms judged to be amenable to both forms of therapy. Among 2143 patients who were en-rolled, 1070 underwent craniotomy and microsurgical repair and 1073 were randomized to endovascular therapy. Outcomes were assessed at 2 months and at 1 year. Among the 1063 treated with coils, 250 patients (23.5%) were dependent (eg, required assistance with activities of daily living) or were deceased at 1 year versus 326 (30.9%) of the 1055 treated surgically. 60 Follow-up angiography revealed incomplete aneurysm obliteration in 34% of aneurysms treated with coil embolization and in 18% of those treated surgically. Some incompletely occluded aneurysms will require retreatment, though indications for and the risks of this remain somewhat unclear. A slight increased risk of rebleeding was noted in aneurysms that had been treated with coil embolization. However, a persistent survival benefit at 7 years was noted in the group who received endovascular therapy. 61 Surgical treatment was associated with a risk of development of epilepsy. Further studies must be undertaken to assess longer term survival and to assess cognitive outcomes in the 2 groups.
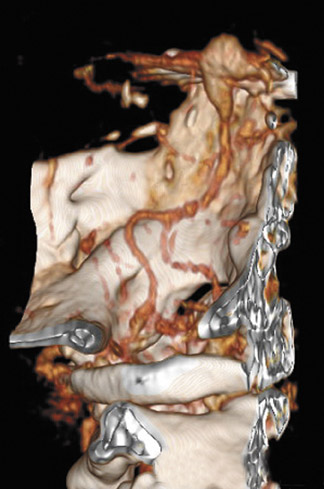
Morbidity and mortality rates associated with endovascular aneurysm therapy have been reported to be 4% to 10% in most large series. Complications have included intracranial hemorrhage secondary to vascular perforation, infarction, and coil compaction and/or migration. Thromboembolic complications may be immediate or delayed, and ischemic sequelae may be minimized by the use of thrombolysis. Additionally, postprocedural use of antiplatelet agents and/or anticoagulation may reduce the incidence of these thromboembolic phenomena. 60-70 Coil compaction may lead to recanalization of the aneurysm, and coil migration may lead to vascular occlusion with resultant ischemia (Figure 10). Contraindications to coil embolization include origination of branch vessels from the aneurysm, the presence of an associated intraparenchymal hematoma requiring surgical evacuation, and parent vessel stenosis (though this may be treated beforehand). The presence of a wide neck has previously been viewed as a relative contraindication due to the relatively lower success rates of aneurysm obliteration and due to the risk of coil prolapse into the parent vessel. 70 However, these issues have become less significant with more widespread use of adjuvant techniques, particularly deployment of intracranial stents (Figure 71,72 and many aneurysms with wide necks are now treated with endovascular techniques. Despite the improved results cited above, many aneurysms will recanalize. Up to 15% to 30% of totally or subtotally embolized aneurysms will demonstrate recanalization, and approximately 5% of aneurysms will require retreatment. 67-70 As such, careful follow-up is mandatory. The gold standard of follow-up continues to be conventional angiography. However, 3D time-of-flight MRA, 3D gadolinium-enhanced MRA, and MRA at 3T have in some cases demonstrated increased sensitivity for detection of flow within the coil interstices. 73-75
Although stents were, in general, intended to be utilized in conjunction with coils, in some cases they have been used as the sole treatment for certain aneurysms, particularly intra- and extracranially for treatment of dissecting aneurysms and pseudoaneurysms and intracranially for treatment of fusiform aneurysms. 76 Successful occlusion of aneurysms treated in this way likely results from interference of inflow into the aneurysm with subsequent stagnation of flow, thrombosis, fibrosis, and the development of a neointima around the wire mesh.
Despite advances in technology, there are aneurysms that are not amenable to coil embolization, stent placement, or microsurgical repair, and these are best treated with parent vessel occlusion (Figure 12). This is typically performed with coils, though detachable balloons have been used in the past. When feasible, temporary arterial occlusion testing is performed prior to vessel sacrifice. This is typically performed with a nondetachable balloon catheter while the patient is systemically anticoagulated and receiving conscious sedation. Frequent neurologic examinations are performed, and the procedure is immediately terminated if a deficit develops. 77-80 Many adjuvant techniques, including measurement of stump pressures, 81,82 induced hypotension, 83,84 single photon emission tomography (SPECT), 85-89 perfusion imaging, 90-92 xenon CT, 93-95 cerebral blood flow measurements, 96 transcranial Doppler examinations, 97 electroencephalography (EEG), 98-100 and monitoring of somatosensory-evoked potentials 101,102 have been described in attempts to increase the sensitivity of this procedure for the detection of ischemia. Patients who cannot tolerate the test occlusion will require a revascularization procedure prior to parent vessel sacrifice.
Conclusion
The topic of cerebral aneurysms is likely to remain controversial. A number of recent studies have significantly altered many preexisting beliefs about the risks associated with aneurysms and have better stratified those risks based upon specific aneurysm characteristics. Increasingly noninvasive imaging modalities have been used for aneurysm evaluation, and their role is likely to increase with increased utilization of higher field-strength MR and multidetector CT. Rapid technologic advances in the endovascular therapy of aneurysms have made many more aneurysms amenable to catheter-based therapies and have improved the success rates of these therapies.
REFERENCES
- Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: A systematic review. Stroke. 1998;29:251-256.
- McCormick WF, Nofzinger JD. Saccular intracranial aneurysms: An autopsy study. J Neurosurg. 1965:22;155-159.
- Housepian EM, Pool JL. A systematic analysis of intracranial aneurysms from the autopsy file of the Presbyterian hospital, 1914 to 1956. J Neuropathol Exp Neurol. 1958;17:409-423.
- Inagawa T, Hirano A. Autopsy study of unruptured incidental intracranial aneurysms. Surg Neurol. 1990;34:361-365.
- Sugai Y, Hamamoto Y, Ookubo T, So K. Angiographical frequency of unruptured incidental intra-cranial aneurysms. No Shinkei Geka. 1994;22: 429-432.
- Camarata PJ, Latchaw RE, Rüfenacht DA, Heros RC. Intracranial aneurysms. Invest Radiol. 1993;28: 373-382.
- Nehls DG, Flom RA, Carter LP, Spetzler RF. Multiple intracranial aneurysms: Determining the site of rupture. J Neurosurg. 1985;63:342-348.
- Schievink WI, Michels VV, Piepgras DG. Neurovascular manifestations of heritable connective tissue disorders. A review. Stroke. 1994;25:889-903.
- Setton A, Davis AJ, Bose A, et al. Angiography of cerebral aneurysms. Neuroimaging Clin N Am. 1996;6:705-738.
- Leblanc R, Melanson D, Tampieri D, Guttmann RD. Familial cerebral aneurysms: A study of 13 families. Neurosurgery. 1995;37:633-639; discussion 638-639.
- Kasuya H, Onda H, Takeshita M, et al. Clinical features of intracranial aneurysms in siblings. Neurosurgery. 2000;46:1301-1305; discussion 1305-1306.
- Bagley LJ, Hurst RW. Angiographic evaluation of aneurysms affecting the central nervous system. Neuroimaging Clin N Am. 1997;7:721-738.
- Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: A long-term follow-up study. Stroke. 2001;32:485-491.
- Inci S, Spletzler RF. Intracranial aneurysms and arterial hypertension: A review and hypothesis. Surg Neurol. 2000;53:530-540; discussion 540-542.
- King JT Jr. Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin N Am. 1997;7:659-668.
- Okamoto K, Horisawa R, Ohno Y. The relationships of gender, cigarette smoking, and hypertension with the risk of aneurysmal subarachnoid hemorrhage: A case-control study in Nagoya, Japan. Ann Epidemiol. 2005;15:744-748.
- Crowell RM, Ogilvy CS. Traumatic intracranial aneurysms. In Ojemann, RG, Ogilvy CS, Crowell RM, Heros RC: Surgical Management of Neuro vascular Disease. Baltimore,MD: Williams & Wilkins, 1995: 377-384.
- Shimoji T, Bando K, Nakajima K, Ito K. Dissecting aneurysms of the vertebral artery. Report of seven cases and angiographic findings. J Neurosurg. 1984;61:1038-1046.
- Ojemann RG. Infectious intracranial aneurysms. In Ojemann, RG, Ogilvy CS, Crowell RM, Heros RC: Surgical Management of Neurovascular Disease. Baltimore, MD:Williams & Wilkins, 1995: 368-376.
- Pasqualin A, Battaglia R, Scienza R, Da Pian R. Italian Cooperative Study on Giant Intracranial Aneurysms: 3. Modalities of treatment. Acta Neurochir Suppl (Wien). 1988;42:60-64.
- Aletich VA, Debrun GM, Monsein LH, et al. Giant serpentine aneurysms: A review and presentation of five cases. AJNR Am J Neuroradiol. 1995;16:1061-1072. Erratum in: AJNR Am J Neuroradiol. 1995; 16(8):1747.
- Heros RC. Giant aneurysms. In Ojemann, RG, Ogilvy CS, Crowell RM, Heros RC. Surgical Management of Neurovascular Disease. Baltimore, MD: Williams & Wilkins, 1995:323-367.
- Horowitz MB, Yonas H, Jungreis C, Hung TK. Management of a giant middle cerebral artery fusiform serpentine aneurysm with distal clip application and retrograde thrombosis. Case report and review of the literature. Surg Neurol. 1994;41:221-225.
- Mawad ME, Klucznik RP. Giant serpentine aneurysms: Radiographic features and endovascular treatment. AJNR Am J Neuroradiol. 1995;16: 1053-1060.
- Turjman F, Massoud TF, Viñuela F, et al. Aneurysms related to cerebral arteriovenous mal-formation: Superselective angiographic assessment in 58 patients. AJNR Am J Neuroradiol. 1994;15: 1601-1605.
- Berenstein A, Lasjaunias P. Classification of brain arteriovenous malformations. In: Surgical Neuroangiography. Vol 4. Berlin: Springer-Verlag; 1992:1-88.
- Grossman RI, Yousem DM. Vascular diseases of the brain. In: Grossman RI, Yousem DM, eds. Neuroradiology: The Requisites . St. Louis, MO: Mosby-Year Book, Inc.;2003:173-241.
- Locksley HB, Sahs AL, Knowler L. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section II. General survey of cases in the central registry and characteristics of the sample population. J Neurosurg. 1966; 24:922-932.
- Wood EH. Angiographic identification of the ruptured lesion in patients with multiple aneurysms. J Neurosurg. 1964;21:182-198.
- Juvela S, Porras M, Heiskanen O. Natural history of unruptured intracranial aneurysms: A long-term follow-up study. J Neurosurg. 1993;79: 174-182.
- Unruptured intracranial aneurysms-risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339:1725-1733. Erratum in: N Engl J Med. 1999;340:744.
- Wiebers DO, Whisnant JP, Huston J 3rd, et al. International Study of Unruptured Intracranial Aneurysms Investigators. Lancet. 2003;362:103-110.
- Davies KN, Humphrey PR. Complications of cerebral angiography in patients with symptomatic carotid territory ischaemia screened by carotid ultrasound. J Neurol Neurosurg Psychiatry. 1993;56: 967-972.
- Gabrielsen TO. Commentary: Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol. 1994;15:1408-1411.
- Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke. 1990;21:209-222.
- Heisermann JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol. 1994;15:1401-1407; discussion 1408-1411.
- Grossman RI, Yousem DM. Techniques in neuroimaging. In: Grossman RI, Yousem DM, eds. Neuroradiology: The Requisites. St. Louis, Mosby-Year Book, Inc., 2003.
- Kaufmann TJ, Huston J 3rd, Mandrekar JN, et al. Complications of diagnostic cerebral angiography: Evaluation of 19,826 consecutive patients. Radiology. 2007;243:812-819.
- Chappell ET, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: A meta-analysis. Neurosurgery 2003; 52:624-631; discussion 630-631.
- Papke K, Brassel F. Modern cross-sectional imaging in the diagnosis and follow-up of intracranial aneurysms. Eur Radiol. 2006;16:2051-2066.
- Atlas SW, Sheppard L, Goldberg HI, et al. Intracranial aneurysms: Detection and characterization with MR angiography with use of an advanced postprocessing techniquein a blinded-reader study. Radiology. 1997;203:807-814.
- Taschner CA, Thines L, Lernout M, et al. Treatment decision in ruptured intracranial aneurysms: Comparison between multi-detector row CT angiography and digital subtraction angiography. J Neuroradiol. 2007;34:243-249.
- Hoh BL, Cheung AC, Rabinov JD, et al. Results of a prospective protocol of computed tomographic angiography in place of catheter angiography as the only diagnosticand pretreatment planning study for cerebral aneurysms by a combined neurovascular team. Neurosurgery. 2004;54:1329-1340; discussion 1340-1342.
- Papke K, Kuhl CK, Fruth M, et al. Intracranial aneurysms: Role of multidetector CT angiography in diagnosis and endovascular therapy planning. Radiology. 2007;244:532-540.
- Rinkel GJ, Wijdicks EF, Vermeulen M, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR Am J Neuroradiol . 1991; 12:829-834.
- Kershenovich A, Rappaport ZH, Maimon S. Brain computed tomography angiographic scans as the sole diagnostic examination for excluding aneurysms in patients with perimesencephalic subarachnoid hemorrhage. Neurosurgery. 2006; 59:798-801; discussion 801-802. Comment in: Neurosurgery . 2007;61:E1340.
- Kallmes DF, Clark HP, Dix, JE, et al. Ruptured vertebrobasilar aneurysm: Frequency of the nonaneurysmal perimesencephalic pattern of hemorrhage on CT scans. Radiology. 1996;201:657- 660.
- Metens T, Rio F, Balériaux D, et al. Intracranial aneurysms: Detection with gadolinium-enhanced dynamic three-dimensional MR angiography-Initial results. Radiology. 2000;216:39-46.
- Macdonald RL, Wallace MC, Kestle JR. Role of angiography following aneurysm surgery. J Neurosurg. 1993;79:826-832.
- Barrow DL, Boyer KL, Joseph GJ. Intraoperative angiography in the management of neurovascular disorders. Neurosurgery. 1992;30:153-159.
- Drake CG, Friedman AH, Peerless, SJ. Failed aneurysm surgery. Reoperation in 115 cases. J Neurosurg. 1984;61:848-856.
- Lin T, Fox AJ, Drake CG. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J Neurosurg. 1989;70:556-560.
- Martin NA, Bentson J, Viñuela F, et al. Intraoperative digital subtraction angiography and the surgical treatment of intracranial aneurysms and vascular malformations. J Neurosurg. 1990;73:526-533.
- Tang G, Cawley CM, Dion JE, Barrow DL. Intraoperative angiography during aneurysm surgery: A prospective evaluation of efficacy. J Neurosurg. 2002;96:993-999.
- Kallmes DF, Kallmes MH. Cost-effectiveness of angiography performed during surgery for ruptured intracranial aneurysms. AJNR Am J Neuroradiol. 1997;18:1453-62.
- Klopfenstein JD, Spetzler RF, Kim LJ, et al. Comparison of routine and selective use of intraoperative angiography during aneurysm surgery: A prospective assessment. J Neurosurg 2004;100: 230-235.
- King JJ, Berlin J, Flamm E. Morbidity and mortality from elective surgery for asymptomatic, unruptured intracranial aneurysms: Ameta-analysis. J Neurosurg. 1994;81:837-842.
- King JT Jr, Glick HA, Mason TJ, Flamm ES. Elective surgery for asymptomatic, unruptured, intracranial aneurysms: A cost-effectiveness analysis. J Neurosurg. 1995;83:403-412.
- Vindlacheruvu RR, Mendelow AD, Mitchell P. Risk-benefit analysis of the treatment of unruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. 2005;76:234-239.
- Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet. 2002;26; 360:1267-1274.
- Molyneux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366: 809-817.
- Piotin M, Spelle L, Mounayer C, et al. Intracranial aneurysms: Treatment with bare platinum coils-aneurysm packing, complex coils, and angiographic recurrence. Radiology. 2007;243:500-508.
- Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212:348-356.
- Thornton J, Debrun GM, Aletich VA, Bashir Q, Charbel FT, Ausman J. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmidetachable coils. Neurosurgery. 2002;50:239-249.
- Li MH, Gao BL, Fang C, et al. Angiographic follow-up of cerebral aneurysms treated with Guglielmi detachable coils: An analysis of 162 cases with 173 aneurysms. AJNR Am J Neuroradiol. 2006; 27:1107-1112.
- Gallas S, Pasco A, Cottier JP, et al. A multicenter study of 705 ruptured intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol. 2005;26:1723-1731.
- Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: Perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475-482.
- Spelle L, Piotin M, Mounayer C, Moret J. Saccular intracranial aneurysms: Endovascular treatment devices, techniques and strategies, management of complications,results. Neuroimaging Clin N Am. 2006;16:413-451.
- Cognard C, Weill A, Castaings L, et al. Intracranial berry aneurysms: Angiographic and clinical results after endovascular treatment. Radiology. 1998;206:499-510.
- Fernandez-Zubillaga A, Guglielmi G, Viñuela F, Duckwiler GR. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: Correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol. 1994;15:815-820.
- Benitez RP, Silva MR, Klem J, et al. Endovascular occlusion of wide-necked aneurysms with new intracranial microstent (Neuroform) and detachable coils. Neurosurgery. 2004;54:1359-1367.
- Fiorella D, Albuquerque FC, Deshmukh VR, McDougall CG. Usefulness of the Neuroform stent in the treatment of cerebral aneurysms: Results at initial (3-6 mo) follow-up. Neurosurgery. 2005;56: 1191-1201.
- Deutschmann HA, Augustin M, Simbrunner, et al. Diagnostic accuracy of 3D time-of-flight MR angiography compared with digital subtraction angiography for follow-up of coiled intracranial aneurysms: Influence of aneurysm size. AJNR Am J Neuroradiol. 2007;28:628-634.
- Pierot L, Delcourt C, Bouquigny F, et al. Follow-up of intracranial aneurysms selectively treated with coils: Prospective evaluation of contrast-enhanced MR angiography. AJNR Am J Neuroradiol. 2006; 27:744-749.
- Majoie CB, Sprengers ME, van Rooij WJ, et al. MR angiography at 3T versus digital subtraction angiography in the follow-up of intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol. 2005;26:1349-1356.
- Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 2006; 59:291-300.
- Mathis JM, Barr JD, Jungreis CA, et al. Temporary balloon test occlusion of the internal carotid artery: Experience in 500 cases. AJNR Am J Neuroradiol. 1995;16:749-754.
- van der Schaaf IC, Brilstra EH, Buskens E, Rinkel GJ. Endovascular treatment of aneurysms in the cavernous sinus: A systematic review of balloon occlusion of the parent vessel and embolization with coils. Stroke. 2002;33:313-328.
- Vazquez Añon V,Aymard A, Gobin YP, et al. Balloon occlusion of the internal carotid artery in 40 cases of giant intracavernous aneurysm: Technical aspects, cerebral monitoring, and results. Neuroradiology. 1992;34:245-251.
- Meyers PM, Thakur GA, Tomsick TA. Temporary endovascular balloon occlusion of the internal carotid artery with a nondetachable silicone balloon catheter: Analysis oftechnique and cost. AJNR Am J Neuroradiol. 1999;20:559-564.
- Morishima H, Kurata A, Miyasaka Y, et al. Efficacy of the stump pressure ratio as a guide to the safety of permanent occlusion of the internal carotid artery. Neurol Res. 1998;20:732-736.
- Barker DW, Jungreis CA, Horton JA, et al. Balloon test occlusion of the internal carotid artery: change in stump pressure over 15 minutes and its correlation with xenon CTcerebral blood flow. AJNR Am J Neuroradiol. 1993;14:587-590.
- Standard SC, Ahuja A, Guterman LR, et al. Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol. 1995;16:1453-1458.
- Dare AO, Chaloupka JC, Putman CM, et al. Failure of the hypotensive provocative test during temporary balloon test occlusion of the internal carotid artery to predict delayedhemodynamic ischemia after therapeutic carotid occlusion. Surgical Neurology. 1998;50:147-156.
- Tomura N, Omachi K, Takahashi S, et al. Comparison of technetium Tc 99m hexamethylpropyleneamine oxime single-photon emission tomograph with stump pressure during the balloon occlusion test of the internal carotid artery. AJNR Am J Neuroradiol. 2005;26:1937-1942.
- Peterman SB, Taylor A, Hoffman JC Jr. Improved detection of cerebral hypoperfusion with internal carotid balloon test occlusion and 99mTc HMPAO cerebral perfusionSPECT imaging. AJNR Am J Neuroradiol. 1991;12:1035-1041.
- Palestro CJ, Sen C, Muzinic M, et al. Assessing collateral cerebral perfusion with technetium-99m-HMPAO SPECT during temporary internal carotid artery occlusion. J Nucl Med. 1993;34: 1235-11238.
- Ryu YH, Chung TS, Lee JD, et al. HMPAO SPECT to assess neurologic deficits during balloon test occlusion. J Nucl Med. 1996;37:551-554.
- Suguwara Y, Kikuchi T, Ueda T, et al. Usefulness of brain SPECT to evaluate brain tolerance and hemodynamic changes during temporary balloon occlusion test and afterpermanent carotid occlusion. J Nucl Med. 2002;43:1616-1623.
- Michel E, Liu H, Remley KB, et al. Perfusion MR neuroimaging in patients undergoing balloon test occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 2001;22:1590-1596.
- Jain R, Hoeffner EG, Deveikis JP, et al. Carotid perfusion CT with balloon occlusion and acetazolamide challenge test: Feasibility. Radiology. 2004; 231:906-913.
- Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990;14:249-265.
- Kofke WA, Brauer P, Policare R, et al. Middle cerebral artery blood flow velocity and stable xenon-enhanced computed tomographic blood flow during balloon test occlusion of the internal carotid artery. Stroke. 1995;26:1603-1606.
- Johnson DW, Stringer WA, Marks MP, et al. Stable xenon CT cerebral blood flow imaging: Rationale for and role in clinical decision making. AJNR Am J Neuroradiol. 1991;12:201-213.
- Linskey ME, Jungreis CA, Yonas H, et al. Stroke risk after abrupt internal carotid artery sacrifice: Accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994;15:829-843.
- Marshall RS, Lazar RM, Young WL, et al. Clinical utility of quantitative cerebral blood flow measurements during internal carotid artery occlusion tests. Neurosurgery. 2002;50:996-1004; discussion 1004-1005.
- Eckert B, Thie A, Carvajal M, et al. Predicting hemodynamic ischemia by transcranial Doppler monitoring during therapeutic balloon occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 1998;19:577-582.
- Morioka T, Matsushima T, Fujii K, et al. Balloon test occlusion of the internal carotid artery with monitoring of compressed spectral arrays (CSAs) of electroencephalogram. Acta Neurochir (Wien). 1989; 101:29-34.
- Herkes GK, Morgan M, Grinnell V, et al. EEG monitoring during angiographic balloon test carotid occlusion: Experience in sixteen cases. Clin Exp Neurol. 1993;30:98-103.
- Cloughesy TF, Nuwer MR, Hoch D, et al. Monitoring carotid test occlusions with continuous EEG and clinical examination. J Clin Neurophysiol. 1993; 10:363-369.
- Schellhammer F, Heindel W, Haupt WF, et al. Somatosensory evoked potentials: A simple neurophysiological monitoring technique in supra-aortal balloon test occlusions. Eur Radiol. 1998;8:1586-1589.
- Liu AY, Lopez JR, Do HM, et al. Neurophysiological monitoring in the endovascular therapy of aneurysms. AJNR Am J Neuroradiol. 2003;24:1520-1527.