Vestibular schwannomas: A Review
Images
Vestibular schwannomas (VS) are benign tumors of the nerve sheath and the most common tumor in the cerebellopontine angle, accounting for 6-8% of all intracranial tumors and 80% of cerebellopontine angle (CPA) tumors, with an estimated prevalence of 0.02% and mean age of diagnosis at 58 years.1,2,3 The sporadic form of VS makes up > 90% of cases; there is no predominance for the left or right side.4 The remainder predominantly occur in neurofibromatosis type 2 (NF2); one of the diagnostic criteria for which is bilateral VS. NF2 is a rare autosomal dominant multiple neoplasia syndrome, occurring due to mutations in the NF2 gene located on chromosome 22q12, coding for the tumor suppressor Merlin.5 Genetic studies have demonstrated loss of both wild-type copies in the NF2 tumor suppressor gene as a common finding in sporadic VS as well NF2.6
This paper will review the clinical presentation, natural history, and pathology of vestibular schwannomas, as well as discuss the cystic subtypes and provide an overview of imaging diagnosis and measurement techniques.
Clinical presentation
In a study of 1000 patients, Matthies et al found symptomatic cochlear nerve involvement in 95% of patients with two major symptoms present: hearing loss and tinnitus. Vestibular nerve dysfunction was present in 61% of patients, with a common finding of imbalance. Trigeminal nerve disturbances occurred in 17%. Major symptoms included facial numbness (paresthesia), hypoesthesia and pain.7 Hearing loss can be evaluated on audiometry; however, audiometry results do not necessarily correlate with lesion site or tumor size.8
Natural history
A systematic review on the natural history of VS performed by Yoshimoto et al showed a growth rate of 1.2 mm/year. Although there was a large variation in the frequency of tumor enlargement over time (15 to 85%), the overall frequency of tumor growth was 46%, whereas the frequency of tumor regression was 8%.9 A study performed by Hunter et al found 40.8% of tumors demonstrated a growth of >2 mm during a median observation period of 23 months. In addition, factors predicting growth or requirement of intervention were initial size at diagnosis and disequilibrium.10
Histopathology
Historically known as acoustic neuromas, these tumors have been demonstrated not to involve the acoustic (cochlear) nerve in most cases, nor to be of neuroglial origin. Instead, they originate from Schwann cells,11 and they can occur anywhere lateral to the glial-schwannian junction.10
Although commonly described in the literature as arising from the glial-schwannian junction, a systematic review of archival temporal bone collections by Roosli et al showed 40 tumors within the internal auditory canal, and 10 intralabyrinthine. Of those 40, 4 arose from the cochlear nerve and 36 from the vestibular nerve. Twenty-one tumors clearly arose lateral to the glial-schwannian junction, whereas only 3 were located in the medial one third. Of the tumors involving the vestibular nerve, they occurred roughly equally in the superior and inferior divisions as well as their common trunk.11 This is in contrast to a prospective study of 200 consecutive cases that showed the origin of 91.4% of schwannomas to be from the inferior vestibular nerve. This study also reported an increased incidence of involvement of the inferior vestibular nerve corresponding to an increase in tumor size.12
Two different tissue types may be present in vestibular schwannomas: Antoni type A tissue, which is compact and ordered with a palisading architecture, and Antoni type B tissue, which is myxoid and loose.12 Histological studies have shown no correlation between dominance of Antoni A or B tissue in either cystic or solid vestibular schwannomas.14
Cystic vestibular schwannoma
Cystic VSs represent a subtype of VS. Incidence on previous publications has been reported from 5.7% to 48%; however, the former study had the largest cohort, consisting of 773 patients and is therefore most likely closest to the true incidence.15,16 Cystic VSs tend to be large at diagnosis; they can demonstrate rapid and unpredictable growth, with a significantly shorter duration of symptoms.17,18,19. The rapid growth of cystic VS is attributed to expansion of the cystic component. On histological studies, the Ki-67 index of cystic VS is either lower or not significantly different to that of solid VS.18,20 Ki-67 is a nuclear antigen appearing in certain phases of the nuclear cycle and is an indicator of the rate of cell proliferation.21
Benech et al reported an increased incidence of adherence to the brainstem; however, they found no significant difference in other postsurgical complications, such as CSF leak, cranial nerve impairment or symptomatic post-operative hemorrhage.19 In the immediate post-operative period, facial nerve complications exceed that of solid VS. Long-term postsurgical facial nerve outcomes, however, are not significantly different between solid and cystic VS when taking into account the size of the tumor.1,22
Erratic growth of the cystic component following radiosurgery has been reported.23 Therefore, conservative management or radiotherapy may have unfavorable outcomes, leaving surgery as the main therapeutic option.24 Several mechanisms by which the cystic component grows have been proposed: extravasation of serum proteins from impaired blood-tumor barrier; protein secretion from tumor cells; isolated or repeated intratumoral microhemorrhage; hyaline, fatty and mucinous degeneration; and microcystic changes.18
Imaging diagnosis
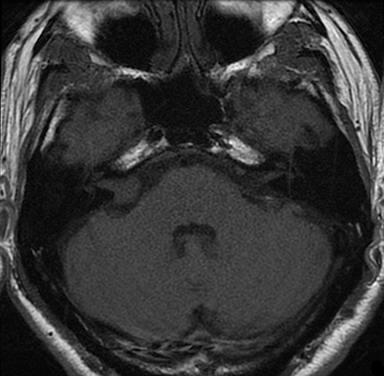
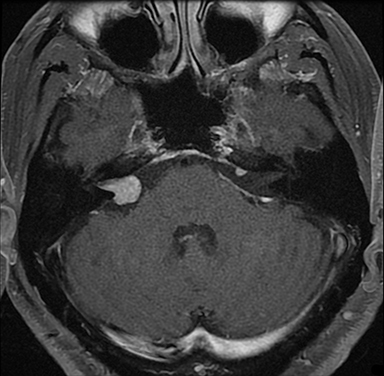
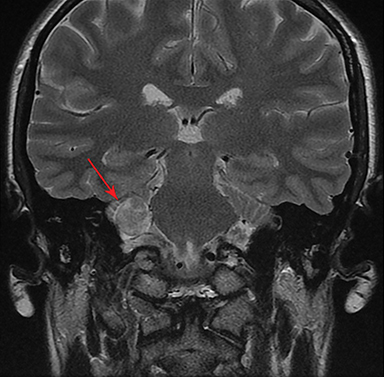
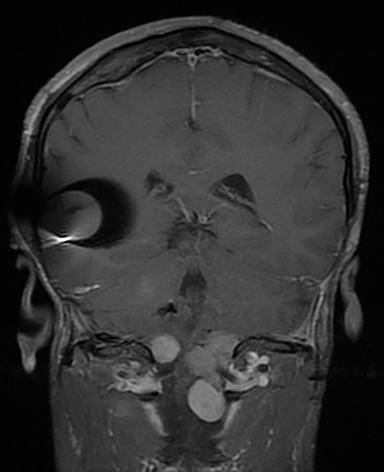
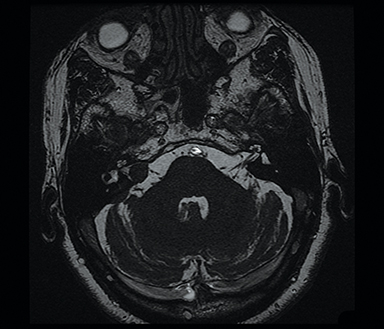
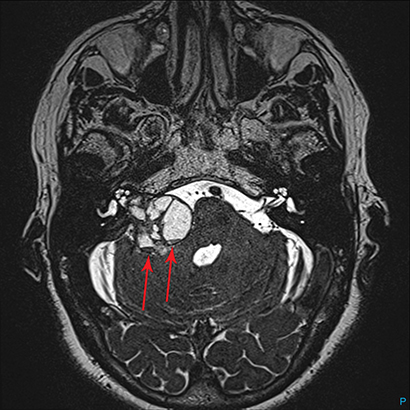
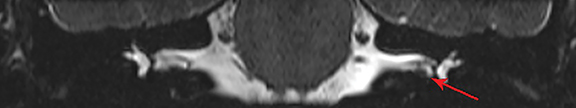
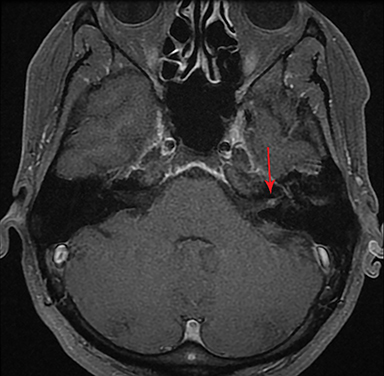
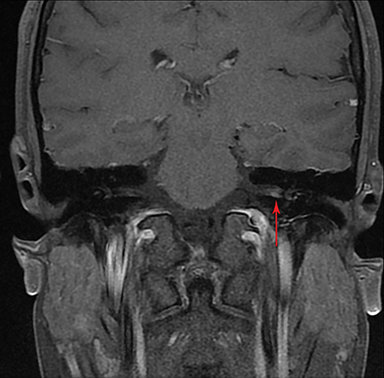
Magnetic resonance imaging (MRI) is the gold standard of imaging diagnosis, owing to excellent soft tissue contrast. Tumors are typically round, ovoid, or lobulated. If there is extension into the cerebellopontine cistern, the angle formed between the tumor and the petrous bone is usually acute. Vestibular schwannomas on MRI are typically T1 isointense or hypointense to adjacent brain tissue and show avid, homogeneous contrast enhancement. Some tumors may show heterogeneous contrast enhancement, which can be due to cystic change.25 On T2 imaging, tumors are most commonly hyperintense, with a small portion showing heterogeneous signal intensity (Figures 1, 2).26 Cystic VS will show T2 hyperintense cysts, which are hypointense on T1W imaging.25 Intratumoral hemorrhage may result in T1 hyperintensity and susceptibility on T2* sequences (Figure 3).3
Heavily T2-weighted sequences such as CISS (Constructive Interference in Steady State, Siemens Healthcare, Erlangen, Germany), SPACE (three-dimensional Sampling Perfection with Application-optimized Contrasts using different flip angle Evolutions, Siemens Healthcare, Erlangen, Germany) and FIESTA-C (Fast Imaging Employing Steady-state Acquisition Cycled Phases) on GE (General Electric, Milwaukee, USA) are commonly used in MR cisternography, offering high contrast between cerebrospinal fluid and adjacent structures.27
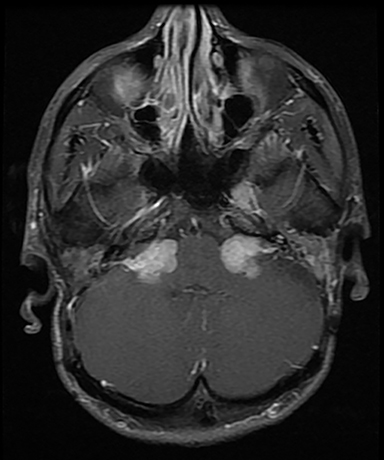
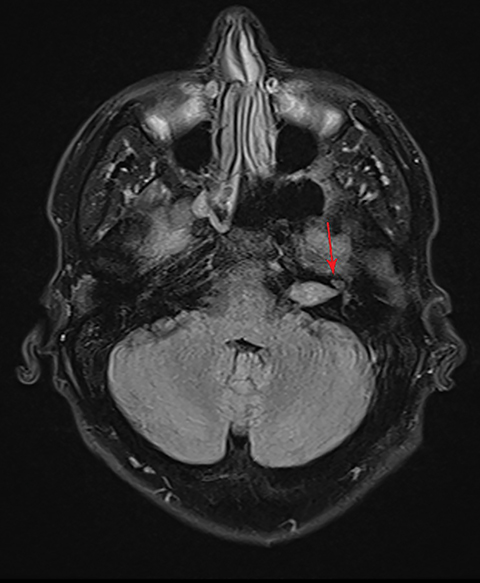
CISS is highly sensitive and specific in the detection of small VS, approaching that of gadolinium-enhanced T1. However, there was an increased risk of missing VS using CISS alone if the tumor was intralabyrinthine in location or < 3 mm in size (Figure 4).28 Despite this limitation, CISS avoids the risks of gadolinium-based contrast agents (GBCA) which are well-known and include hypersensitivity reactions and nephrogenic systemic fibrosis in patients with severe renal dysfunction (glomerular filtration rate < 30 ml/min).29 Additional drawbacks of using GBCA include increased cost and scanning time. On computed tomography (CT) imaging, VS is usually isoattenuating to the adjacent brainstem and demonstrates avid, homogeneous contrast enhancement. The cystic variant will show hypoattenuating cysts, which do not enhance. CT also offers superior assessment of bony anatomy, which can reliably appreciate widening of the internal auditory canal.
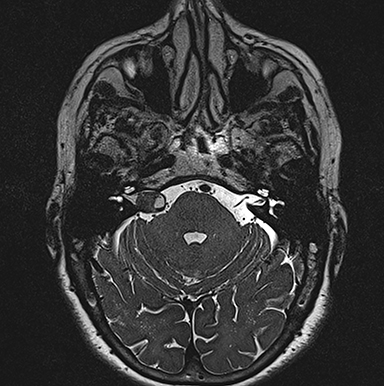
Asymmetric increased ispsilateral cochlear fluid attenuation inversion recovery (FLAIR) signal relative to the brainstem is a known association, attributed to concentration of protein in the perilymphatic space (Figure 5).30,31 Furthermore, relative cochlear signal abnormality tends to be greater in tumors of the CPA compared with purely intrameatal tumors.32
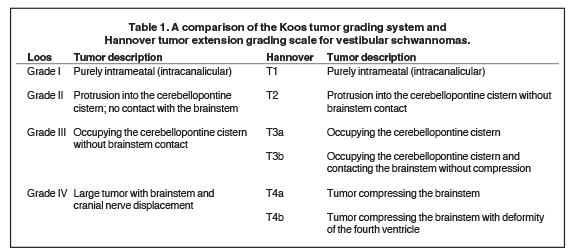
Commonly used tumor grading systems are the Koos grading system and Hannover tumor extension grading system (Table 1). Koos grade I – IV describes tumor size and location from being small and purely intrameatal through to large tumors causing displacement of the brainstem.33 The Hannover tumor extension grading system also adds criteria that include CPA extension with and without brainstem contact (T3a and T3b) and brainstem compression with and without fourth ventricular deformity (T4a and T4b, Table 1).34
Some authors have proposed a classification system for the cystic appearance; eg, Piccirillo et al, who describe a system differentiating between central and thick-walled cysts (Type A) and peripheral and thin-walled cysts (Type B).35 Other groups have classified cysts as either multiple, large ,thin-walled cysts; multiple thick-walled cysts; a single, large, thin-walled cyst; a large, centra,l thick-walled cyst; or a mixed pattern of small and large cysts.22 No single classification system has been widely implemented at the time of this review.
Reporting measurements
Measuring VSs can be challenging, as these tumors often have an irregular shape, and linear measurements can suffer considerable interobserver variability. On follow-up studies, variation in scan technique and plane can also result in a miscalculation of growth rate.
The Consensus Meeting on Systems for Reporting Results in Vestibular Schwannoma described a standardized measurement technique which includes the following: 36
- Size based on measurements in millimeters instead of volumetric measurements.
- Preoperative measurements based on MRI or CT if MRI is unavailable.
- Intrameatal and extrameatal portion of the tumor to be clearly distinguished.
- Only the maximum diameter of the extrameatal portion to be used.
- If the tumor is purely within the internal auditory canal, without extension through the porus acusticus, the term intrameatal should be used.
- It should be noted whether or not the fundus is empty or the tumor is cystic – if single or multiple cysts are present.
- The intrameatal tumor can be measured along the length of the internal auditory canal. The width is measured using a line perpendicular to the above. The size of the intrameatal portion should not be added to the extrameatal tumor size.
Varughese et al assessed growth of VS using a mm/year-based model, cm3/year-based model, and a volume-doubling time (VDT) based-model, concluding the VDT-based model was the most accurate in describing VS growth.37 A literature review by Li et al also concluded linear measurement models are subject to reduced interobserver agreement and provide a poor estimation of tumor volume.38 Volumetric measurements therefore have the greatest clinical utility, sensitivity and accuracy in measuring tumor size and growth.
Conclusion
Vestibular schwannomas are the most common cerebellopontine angle tumor, commonly presenting with a constellation of symptoms related to cranial nerve deficits such as hearing loss. Most are slow growing; however, the cystic variant tends to have a rapid and unpredictable growth pattern, necessitating surgical management rather than a conservative approach. On MRI, VSs are typically T1 isointense, T2 hyperintense, and avidly enhance with contrast. Heavily T2-weighted sequences offer high sensitivity and specificity in the detection of VS, unless the tumors are < 3mm in size or intralabyrinthine in location. The Koos or Hannover tumor extension grading system describe tumor extension from an intracanalicular location through to a large tumor compressing the fourth ventricle. Measurement techniques for VS and a system for classification of the cysts in the cystic variant have not been widely adopted at the time of this review.
References
- Jones S, Baguley D, Moffat D. Are facial nerve outcomes worse following surgery for cystic vestibular schwannoma? Skull Base. 2007;17(5):281-284.
- Lin D, Hegarty J, Fischbein N, et al. The prevalence of “incidental” acoustic neuroma. Arch Otolaryngol Head Neck Surg. 2005;131(3):241.
- Stangerup S, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am. 2012;45(2):257-268.
- Lin E, Crane B. The management and imaging of vestibular schwannomas. AJNR Am J Neuroradiol. 2017;38(11):2034-2043.
- Halliday J, Rutherford S, McCabe M, et al. An update on the diagnosis and treatment of vestibular schwannoma. Expert Rev Neurother. 2017;18(1):29-39.
- Lee J, Kwon T, Kim U, et al. Genetic and epigenetic alterations of the NF2 gene in sporadic vestibular schwannomas. PLoS ONE. 2012;7(1):e30418.
- Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997;40(1):1-10.
- Tutar H, Duzlu M, Göksu N, et al. Audiological correlates of tumor parameters in acoustic neuromas. Eur Arch Otorhinolaryngol. 2012;270(2):437-441.
- Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg. 2005;103(1):59-63.
- Hunter J, Francis D, O’Connell B, et al. Single institutional experience with observing 564 vestibular schwannomas. Otology & Neurotology. 2016;37(10):1630-1636.
- Roosli C, Linthicum, Jr. F, Cureoglu S, et al. What is the site of origin of cochleovestibular schwannomas? Audiol Neurootol. 2012;17(2):121-125.
- Khrais T, Romano G, Sanna M. Nerve origin of vestibular schwannoma: a prospective study. J Laryngol Otol. 2007;122(02).
- Antoni NRE. Über Rückenmarkstumoren und Neurofibrome. Weisbaden, Germany: Bergmann; 1920; 234–311.
- Park C, Kim D, Park S, et al. Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J Neurosurg. 2006;105(4):576-580. doi:10.3171/jns.2006.105.4.576.
- Fundová P, Charabi S, Tos M, et al. Cystic vestibular schwannoma: surgical outcome. J Laryngol Otol. 2000;114(12):935-939.
- Jeng CM, Huang JS, Lee WY, et al. Magnetic resonance imaging of acoustic schwannomas. J Formos Med Assoc. 1995; 94(8):487–493.
- Wandong S, Meng L, Xingang L, et al. Cystic acoustic neuroma. J Clin Neurosci. 2005;12(3):253-255.
- Charabi S, Klinken L, Tos M, et al. Histopathology and growth pattern of cystic acoustic neuromas. Laryngoscope. 1994;104(11):1348-1352.
- Benech F, Perez R, Fontanella M, et al. Cystic versus solid vestibular schwannomas: a series of 80 grade III–IV patients. Neurosurg Rev. 2005;28(3):209-213.
- Park C, Kim D, Park S, et al. Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J Neurosurg. 2006;105(4):576-580.
- Niemczyk K, Vaneecloo F, Lecomte M, et al. Correlation between Ki-67 index and some clinical aspects of acoustic neuromas (vestibular schwannomas). Otolaryngol Head Neck Surg. 2000;123(6):779-783.
- Metwali H, Samii M, Samii A, et al. The peculiar cystic schwannoma: A single-center experience. World Neurosurg. 2014;82(6):1271-1275.
- Pendl G, Ganz J, Kitz K, et al. Acoustic neurinomas with macrocysts treated with gamma knife radiosurgery. Stereotact Funct Neurosurg. 1996;66(1):103-111.
- Nair S, Baldawa S, Gopalakrishnan C, et al. Surgical outcome in cystic vestibular schwannomas. Asian J Neurosurg. 2016;11(3):219.
- Mulkens T, Parizel P, Martin J, et al. Acoustic schwannoma: MR findings in 84 tumors. AJR Am J Roentgenol. 1993;160(2):395-398.
- Singh K, Singh M, Thukral C, et al. Role of magnetic resonance imaging in evaluation of cerebellopontine angle schwannomas. Indian J Otolaryngol Head Neck Surg. 2014;67(1):21-27.
- Ozgen B, Oguz B, Dolgun A. Diagnostic accuracy of the constructive interference in steady state sequence alone for follow-up imaging of vestibular schwannomas. AJNR Am J Neuroradiol. 2009;30(5):985-991.
- Liudahl A, Davis A, Liudahl D, et al. Diagnosis of small vestibular schwannomas using constructive interference steady state sequence. Laryngoscope. 2018. Epub ahead of print.
- Tsushima Y, Kanal E, Thomsen H. Nephrogenic systemic fibrosis: risk factors suggested from Japanese published cases. Br J Radiol. 2010;83(991):590-595.
- Bhadelia R, Tedesco K, Hwang S, et al. Increased cochlear fluid-attenuate inversion recovery signal in patients with vestibular schwannoma. AJNR Am J Neuroradiol. 2008;29(4):720-723.
- Palva T, Raunio V. Cerebrospinal fluid and acoustic neurinoma specific proteins in perilymph. Acta Otolaryngol. 1982;93(1):201-203.
- Kim D, Lee J, Goh M, et al. Clinical significance of an increased cochlear 3D fluid-attenuated inversion recovery signal intensity on an MR imaging examination in patients with acoustic neuroma. AJNR Am J Neuroradiol . 2014;35(9):1825-1829.
- Koos W, Day J, Matula C, et al. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg. 1998;88(3):506-512.
- Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40(1):11–23.
- Piccirillo E, Wiet M, Flanagan S, et al. Cystic vestibular schwannoma. Otol Neurotol. 2009;30(6):826-834.
- Kanzaki J, Tos M, Sanna M. et al. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24(4):642-649.
- Varughese J, Breivik C, Wentzel-Larsen T, et al. Growth of untreated vestibular schwannoma: a prospective study. J Neurosurg. 2012;116(4):706-712.
- Li D, Tsimpas A, Germanwala A. Analysis of vestibular schwannoma size: A literature review on consistency with measurement techniques. Clin Neurol Neurosurg. 2015;138:72-77.
Citation
L NDDK.Vestibular schwannomas: A Review. Appl Radiol. (3):22-27.
June 7, 2019