Untangling the Postoperative Upper GI Tract: Imaging Appearance of Altered Anatomy and Its Complications
CME/CE for this article can be found here.
Introduction
There are many surgical procedures involving the upper gastrointestinal (UGI) tract that result in altered anatomy, such as new alimentary conduits and/or enteric, biliary, or pancreatic anastomoses. These are performed for a variety of indications, which include bariatric and metabolic surgery, malignancy resection, biliary disease, chronic pancreatitis, and reconstruction following injury.
As radiologists play a critical role in assessing postoperative complications, their understanding of postsurgical anatomy is essential for appropriate image interpretation and subsequent patient management. This article reviews common UGI operations, demonstrates their expected postoperative imaging appearance, and highlights pertinent complications.
UGI Procedures That Alter Anatomy
Bariatric Surgery
Roux-en-Y Gastric Bypass
The Roux-en-Y gastric bypass (RYGB) is performed for the treatment of obesity and its associated disorders.1 As its applications have grown, the number of surgeries performed has increased.2
Technically, the RYGB involves stapling the stomach into a gastric pouch and remnant stomach; creating a biliopancreatic limb in continuity with the remnant stomach and duodenum; anastomosis of the biliopancreatic limb to the jejunum (jejunojejunostomy); and restoring the UGI tract by anastomosing the gastric pouch to the jejunum (gastrojejunostomy).3
When identifying an alimentary limb on CT, it helps to work proximally from the gastroesophageal junction and administer oral contrast ( Figure 1 ). Searching for the luminal staple line on multiple planes can help identify the jejunojejunostomy, which can vary in location.
Contrast-enhanced axial CT with oral contrast demonstrates normal postsurgical anatomy of Roux-en-Y gastric bypass (RYGB) ( A ) with the formation of the gastric pouch and alimentary limb (brown arrow) and excluded remnant stomach and afferent limb (yellow arrow). Contrast-enhanced axial CT ( B ) demonstrates pneumoperitoneum (white arrow) in a patient with a remote history of RYGB (brown and yellow arrows). The patient underwent laparotomy and Graham patch repair of a perforation at the gastrojejunal anastomosis. Contrast-enhanced coronal CT reformation of a patient with prior RYGB ( C ) demonstrates small bowel obstruction with dilated and fecalized small bowel (orange arrow), mesenteric swirling (red arrow), and edema (blue arrow). The patient underwent diagnostic laparotomy, which confirmed a perforated Petersen space internal hernia. Contrast-enhanced axial CT with oral contrast of a patient with prior RYGB ( D ) demonstrates mesenteric swirling (red arrows) and edema (blue arrow); small bowel obstruction was present (not shown). Ascending and transverse colon dilation was also noted (pink arrow). The patient underwent diagnostic laparoscopy, which confirmed an internal hernia through the jejunojejunostomy mesenteric defect. Upper gastrointestinal fluoroscopy in a patient with a prior RYGB ( E ) demonstrates an abnormal connection (black arrow) between the gastric pouch (brown arrow) and excluded stomach (yellow arrow). Endoscopy confirmed the presence of a 1.5 cm gastro-gastric fistula. Contrast-enhanced coronal CT reformation in a patient with prior RYGB ( F ) demonstrates small bowel obstruction secondary to jejunojejunal intussusception (purple arrows). Diagnostic laparoscopy demonstrated large segment common channel intussusception, which was successfully reduced and treated with enteroplication of the biliopancreatic limb to the common channel.
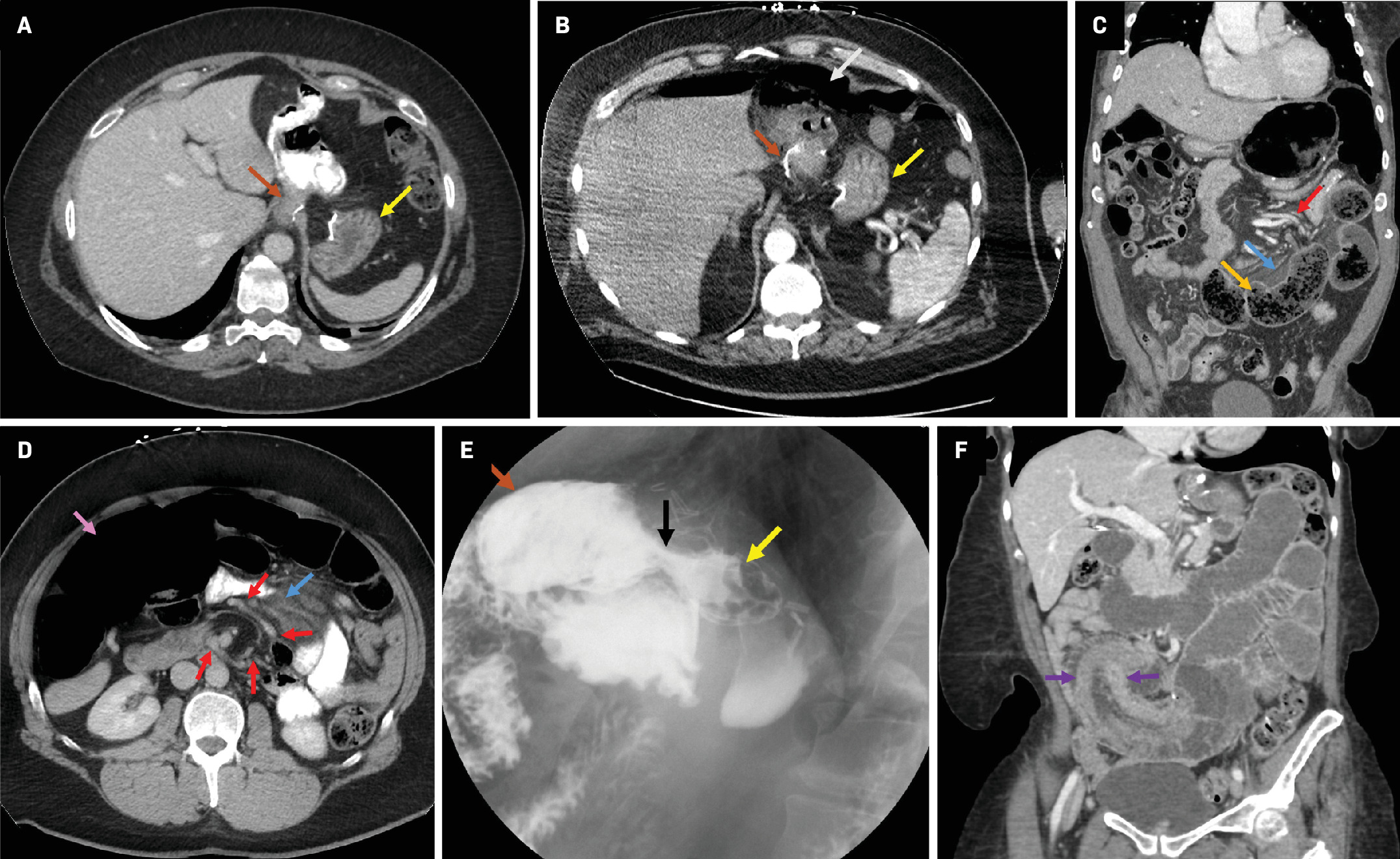
Complications associated with RYGB surgery can occur immediately or later in the postoperative period ( Table 1 ).4 Perforation, a notable late-stage complication, is typically the complication of an ulcer ( Figure 1 ).4 Internal hernias are infrequent and result from migration of bowel loops into mesenteric defects (e.g., Petersen space) created during surgery ( Figure 1 ). Common findings include small bowel obstruction with focal transition points, crowding and abnormal location of small bowel loops, and mesenteric abnormalities such as swirling, engorgement, and stretching of mesenteric vessels.5 Gastro-gastric fistula, which can have early or late onset, should be considered when patients have postoperative weight gain ( Figure 1 ).6
Upper Gastrointestinal Tract Surgeries, Anastomoses, and Complications
| Surgery | Anastomoses | Complications |
|---|---|---|
| Roux-en-Y gastric bypass | Gastrojejunostomy Jejunojejunostomy |
Early Anastomotic leak Gastro-gastric fistula Hemorrhage Intra-abdominal infection Roux-en-O error Stenosis Late Afferent loop syndrome Gastro-gastric fistula Internal hernia Intussusception Marginal ulcer Obstruction Perforation |
| Duodenal switch | Duodenoileostomy Ileoileostomy |
Afferent loop syndrome Anastomotic leak Bowel obstruction Duodenal stump leak Hemorrhage Internal hernia Stenosis |
| Nissen, Toupet, or Dor fundoplication | N/A | Gastric/esophageal leak Stomach slippage Tight wrap Wrap disruption Wrap migration |
| Billroth I | Gastroduodenostomy | Anastomotic leak Bowel obstruction Hemorrhage Intra-abdominal infection Marginal ulcer Reflux |
| Billroth II | Gastrojejunostomy ± BEE |
Afferent loop syndrome Anastomotic leak Anastomotic stricture Bowel obstruction Duodenal stump leak Efferent loop syndrome Gastric adenocarcinoma Hemorrhage Internal hernia Intra-abdominal infection Marginal ulcer |
| Total gastrectomy | Esophagojejunostomy Jejunojejunostomy |
Afferent loop syndrome Anastomotic leak Bowel obstruction Hemorrhage Internal hernia Intra-abdominal infection |
| Whipple | Duodeno- or gastrojejunostomy Hepaticojejunostomy Pancreaticojejunostomy or pancreaticogastrostomy ± BEE |
Afferent loop syndrome Anastomotic leak Bile leak Pancreatic fistula Anastomotic stricture Delayed gastric emptying Hemorrhage GDA pseudoaneurysm Intra-abdominal infection Marginal ulcer Pancreatitis |
| Total pancreatectomy | Gastrojejunostomy Hepaticojejunostomy |
Anastomotic leak Bile leak Anastomotic stricture Hemorrhage Intra-abdominal infection Marginal ulcer |
| Puestow procedure | Jejunojejunostomy Pancreaticojejunostomy |
Anastomotic leak Bowel obstruction Hemorrhage Intra-abdominal infection |
| Roux-en-Y hepaticojejunostomy or choledochojejunostomy | Hepaticojejunostomy and jejunojejunostomy Choledochojejunostomy |
Anastomotic leak Bile leak Anastomotic stricture Cholangitis Intra-abdominal infection Sump syndrome |
Abbreviations: BEE, Braun enteroenterostomy; GDA, gastroduodenal artery.
Less common complications include the Roux-en-O error in which the biliopancreatic limb is connected to the gastric pouch, creating a continuous, obstructive circuit.7 Afferent loop syndrome, an infrequent condition that results from an isolated obstruction of the biliopancreatic limb,8 should be differentiated from typical obstruction, as it may warrant a follow-up endoscopic evaluation of the jejunojejunostomy.9 Retrograde intussusception, while rare, usually presents with symptoms of bowel obstruction ( Figure 1 ).10
Duodenal Switch
The duodenal switch (DS), also known as biliopancreatic diversion with duodenal switch (BPD-DS), is less common than RYGB but it has seen a recent renaissance.2 This approach combines a sleeve gastrectomy (SG) with diversion of bile and pancreatic enzymes, which leads to fat malabsorption and weight loss.11 This can be performed as a primary operation or for patients who have experienced weight gain after SG alone as it is sometimes safer than a repeat SG or RYGB revision.12
The procedure consists of SG; transection of the duodenum and ileum to create the biliopancreatic limb; duodenoileostomy to restore continuity of the UGI tract and create the alimentary limb; and ileoileostomy to anastomose the biliopancreatic limb to the alimentary limb.12
Strategies to identify the alimentary limb are similar to those used for RYGB. The duodenal stump of the biliopancreatic limb can usually be identified by a surgical staple line near the porta hepatis. As with RYGB, the intra-abdominal location of the ileoileostomy can vary ( Figure 2 ).
Contrast-enhanced coronal CT reformation with oral contrast demonstrates the normal appearance of a duodenal switch, with sleeve gastrectomy (brown arrow), biliopancreatic limb (green arrows), and ileoileal anastomosis (yellow arrow).
Complications associated with BPD-DS are analogous to those for RYGB ( Table 1 ).13, 14 In particular, the duodenal stump can leak. Clues to this diagnosis include fluid or free air adjacent to the staple line. Diagnosing duodenal stump leak using CT with oral contrast or fluoroscopic studies can be challenging as the peristaltic wave of the biliopancreatic limb toward the ileoileostomy hinders retrograde flow of contrast along the long luminal channel. Additionally, afferent loop syndrome is possible, although rare.15
Anti-Reflux Surgery
Fundoplication
Gastroesophageal reflux disease is common, affecting an estimated 20% of North Americans.16 Nissen fundoplication is a popular, antireflux surgery used with patients who have refractory symptoms despite medical therapy.17 The technical elements include mobilization of the distal esophagus into the abdomen and suturing the proximal stomach in a 360° wrap around the esophagus, which contains a bougie dilator to maintain patency.18 A partial 270° posterior wrap (Toupet) or anterior 180° wrap (Dor) are performed in some instances, such as when there is underlying esophageal dysmotility.16
On CT, the fundoplication wrap normally appears as a swirling, focal bulge below the esophageal diaphragmatic hiatus and can contain oral contrast ( Figure 3 ).18 Fluoroscopy will demonstrate narrowing of the distal esophagus and a smooth filling defect.18
Contrast-enhanced coronal CT reformation ( A ) demonstrates the normal appearance of a Nissen fundoplication, with a swirling bulge located just below the esophageal diaphragmatic hiatus (yellow arrow). Upper gastrointestinal fluoroscopy ( B ) demonstrates partial unwrapping of a Nissen fundoplication, which contains oral contrast within the wrap (white arrows) and is located above the diaphragm.
Complications are predominantly related to wrap formation and integrity ( Table 1 ).18 Patients can present with symptoms of recurrent reflux, obstruction, or both, which are typically evaluated with fluoroscopy ( Figure 3 ).18 Intraoperative gastric or esophageal perforations, while rare, have been reported.19
Gastrectomy
Billroth I and Billroth II
The Billroth I (BI) reconstruction is used to resect a malignancy or an ulcer following a distal gastrectomy. It is an end-to-end reconstruction of the alimentary tract, involving a gastroduodenostomy between the remnant stomach and duodenum.
The Billroth II (BII) reconstruction, an alternative procedure, is applied more frequently. Rather than directly re-anastomosing the duodenum and remnant stomach, the BII entails stapling the proximal duodenum to create a duodenal stump near the Ampulla of Vater.20 A gastrojejunostomy is then performed to restore continuity of the GI tract.20 The afferent limb may be formed in either an iso- or anti-peristaltic configuration. A variation using a Roux limb for the gastrojejunostomy, which involves the creation of a jejunojejunostomy distal to the gastrojejunostomy, has grown in popularity.21 Furthermore, a Braun enteroenterostomy (BEE), an “omega loop” forming a side-to-side jejunojejunostomy that diverts retrograde reflux, is sometimes added to the procedure.22
On CT, following the esophagus distally to the remnant stomach will help identify the gastrojejunostomy ( Figure 4 ). The duodenal stump staple line is similarly identified adjacent to the liver ( Figure 4 ).
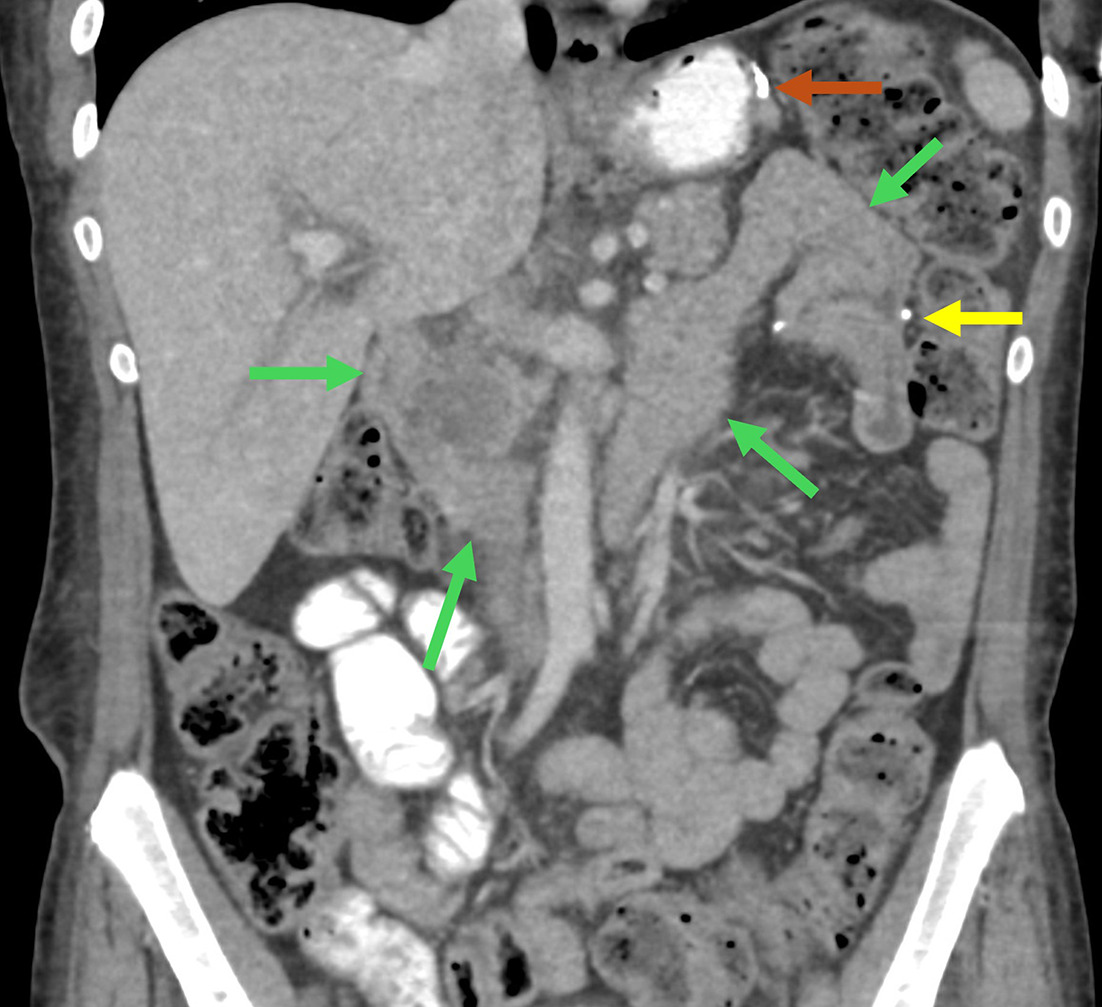
Coronal CT reformation ( A ) demonstrates normal appearance of a Billroth II gastrojejunostomy (brown arrow) and duodenal stump (green arrow). Contrast-enhanced coronal CT reformation with oral contrast of a patient with leukocytosis ( B ) on postoperative day 3 following gastrectomy with Billroth II reconstruction. There is a right upper-quadrant fluid collection (blue arrows) abutting the duodenal stump (green arrow). Percutaneous drainage was performed, which removed 375 mL of bilious fluid, suspicious for a duodenal stump leak. Contrast-enhanced coronal CT reformation of a patient with prior Billroth II reconstruction ( C ) presenting with nausea and vomiting. There is dilation of the biliopancreatic limb (yellow arrows) with normal caliber of the efferent limb (brown arrow) consistent with afferent limb syndrome. The patient’s symptoms and imaging findings resolved with nasogastric tube decompression. Follow-up endoscopy was unremarkable.
The complication profiles of BI and BII are similar ( Table 1 ).23 - 25 Internal hernia is only possible following BII, especially with Roux limb variants.26 As in BPD-DS, the BII duodenal stump can, rarely, be the source of an anastomotic leak ( Figure 4 )27 and afferent loop syndrome is possible ( Figure 4 ).28 Even more rare is efferent loop syndrome, which is obstruction at the gastrojejunostomy from edema or limb kinking in the early postoperative period, which presents with symptoms of bowel obstruction.29
Total Gastrectomy
Total gastrectomy is performed when gastric malignancy affects the proximal stomach, and a Roux-en-Y esophagojejunostomy is the preferred method for reconstruction.20 The process involves transection of the distal esophagus and duodenum, creating the duodenal stump and biliopancreatic limb; transection of the jejunum and re-anastomosis with esophagojejunostomy, creating the alimentary limb; and jejunojejunostomy to anastomose the biliopancreatic and alimentary limbs. Strategies for identifying these limbs on imaging are similar to those previously discussed.
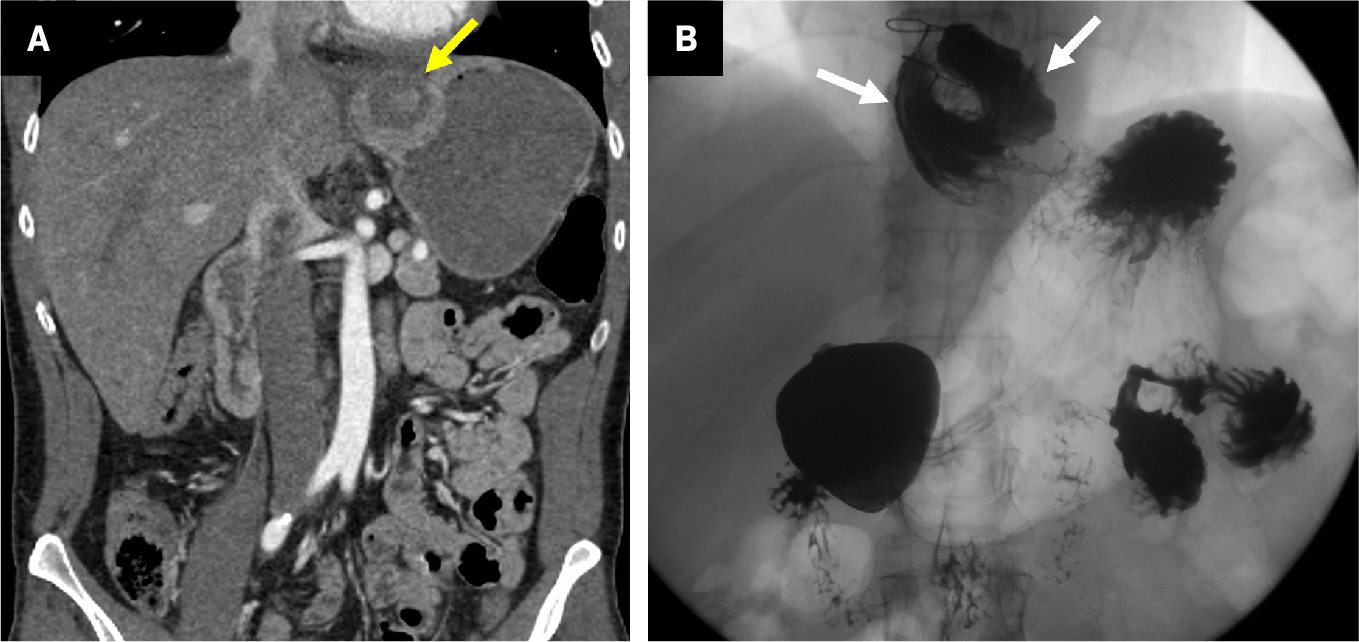
Postoperative complications are similar to those for BII ( Table 1 ). One feared complication is leakage of the esophagojejunal anastomosis ( Figure 5 ).30 The presence of fluid or free air adjacent to the anastomosis or new pleural/mediastinal effusion should raise suspicion of a leak.
Contrast-enhanced coronal CT reformation of a patient with recent total gastrectomy demonstrates air (white arrows) and fluid (blue arrow) adjacent to the esophagojejunal anastomosis along with a left pleural effusion (purple arrow) concerning for leak. Anastomotic leak was confirmed at laparotomy and treated with esophageal stent placement.
Hepatopancreatobiliary Surgery
Whipple
The Whipple procedure, also called pancreaticoduodenectomy, is performed for malignant and benign diseases of the pancreatic head.31 The technique comprises resection of the pancreatic head, duodenum, and proximal jejunum; cholecystectomy; pancreaticojejunostomy, or less commonly pancreaticogastrostomy; duodenojejunostomy (in pylorus-preserving Whipple) or gastrojejunostomy; and hepaticojejunostomy. Optional BEE has also demonstrated lower rates of delayed gastric emptying and afferent loop syndrome ( Figure 6 ).32
Contrast-enhanced coronal CT reformation with oral contrast demonstrates the normal appearance of a Whipple with gastrojejunostomy (brown arrows) and additional side-to-side Braun enteroenterostomy (yellow arrows).
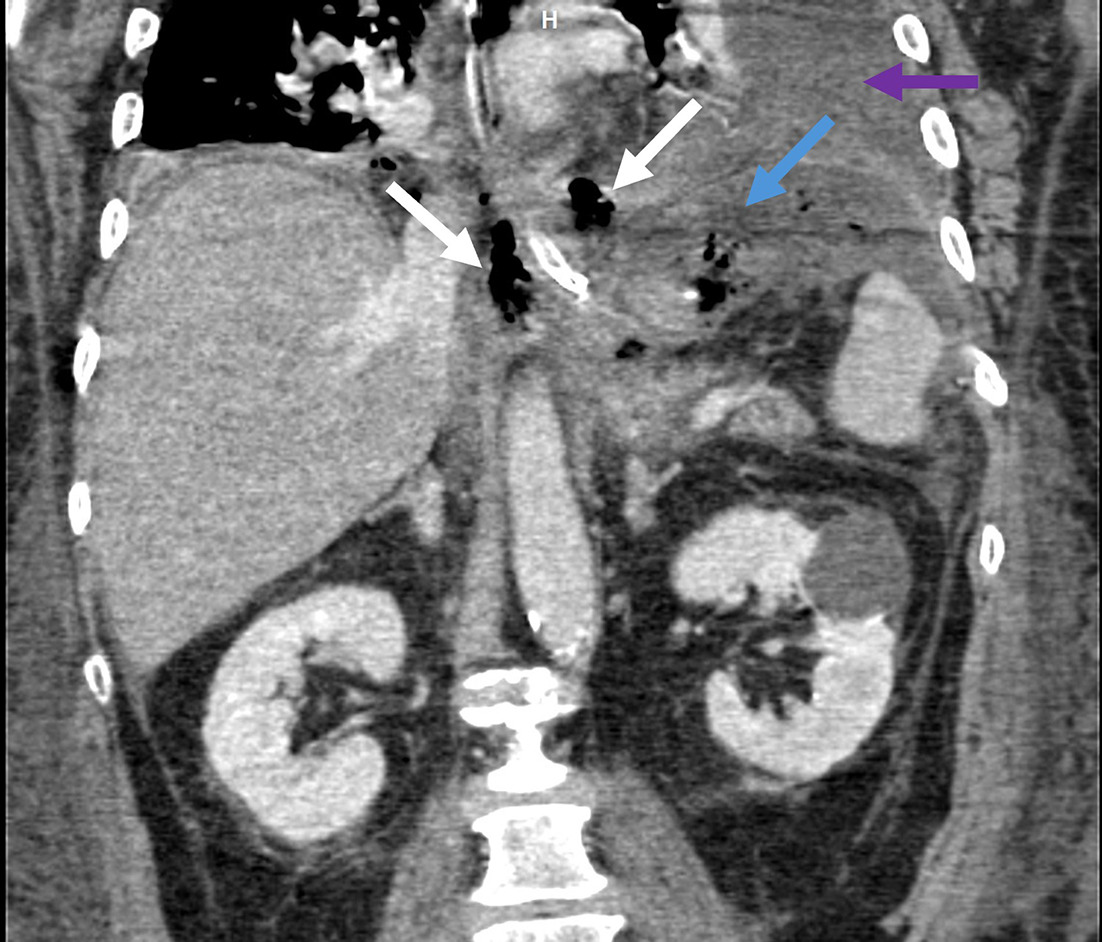
Identifying the anastomosis following a pancreaticojejunostomy is aided by identifying the remnant pancreas in the native bed and then searching distally. Alternatively, searching backward a few centimeters from the jejunal end, often delineated with a staple line, will lead to the pancreaticojejunostomy site. A pancreaticogastrostomy will be visible following the stomach distally from the gastroesophageal junction. Determining the location of the common hepatic duct and proximal common bile duct (CBD) is useful before moving distally to the hepaticojejunostomy, and the presence of pneumobilia can aid in detection.33 Lastly, following the stomach distally from the gastroesophageal junction will help identify the duodenojejunostomy/gastrojejunostomy, most often now in a left upper-quadrant, antecolic position.
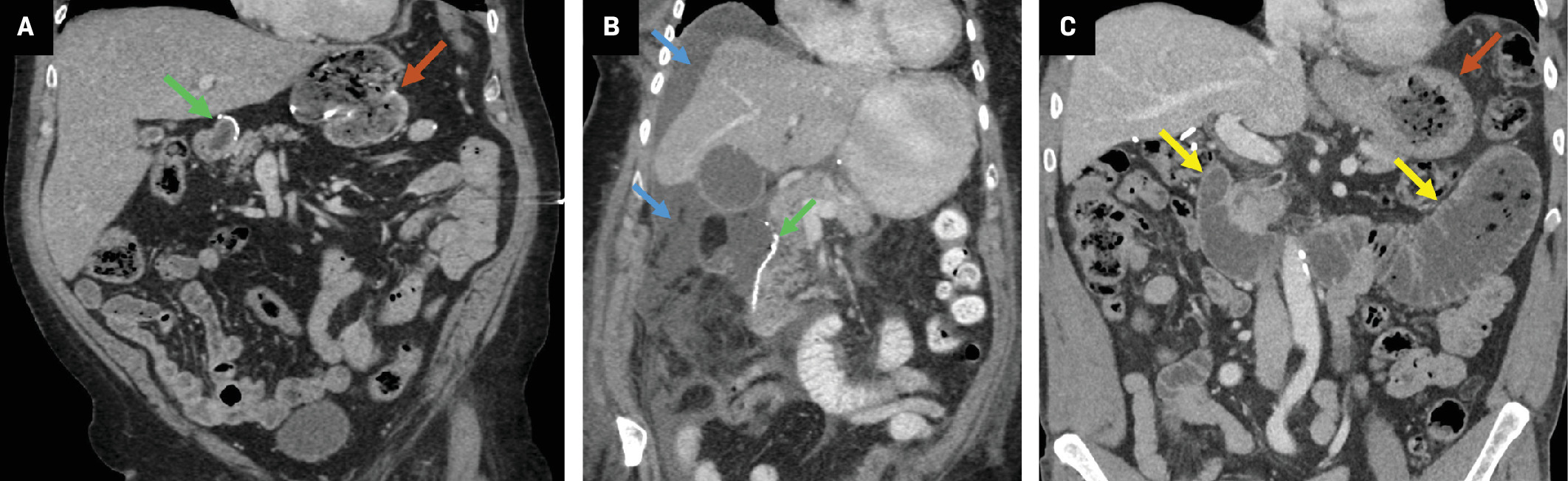
The postoperative complications from the Whipple procedure are summarized in Table 1 .31 Postoperative pancreatic fistula (POPF), or leakage of pancreatic enzyme-containing fluid from the pancreaticojejunostomy ( Figure 7 ), is a hazardous complication that has received great attention in surgical literature.34 One challenge in diagnosing POPF on imaging is differentiating between benign, reactive peripancreatic postsurgical fluid and fluid stemming directly from pancreatic leak. Helpful clues for diagnosis are the presence of extraluminal air or surrounding inflammatory changes, clinical exam findings, and surgical drain output parameters.31, 35 Furthermore, the presence of fluid adjacent to the hepaticojejunostomy can be indicative of a bile leak; however, distinguishing this from a POPF can be challenging on CT.31 If suspected, a nuclear medicine hepatobiliary iminodiacetic acid (HIDA) scan can be a helpful adjunct. In the long term, stricture may develop at the hepaticojejunostomy, resulting in jaundice or cholangitis, and this can be assessed with magnetic resonance cholangiopancreatography (MRCP, Figure 7 ).31
Contrast-enhanced coronal CT reformation (A) on postoperative day 3 following a Whipple. There is a biliary stent and a fluid collection extending into the left upper-quadrant (blue arrows), which abuts the pancreaticojejunostomy (orange arrow). Percutaneous drainage removed 100 mL of gray fluid with amylase of 7915 and lipase > 300,000, consistent with pancreatic fistula. Coronal maximum intensity projection (MIP) image from 3D magnetic resonance cholangiopancreatography in a patient with prior Whipple ( B ) and symptoms of biliary obstruction demonstrates an abrupt cutoff and signal loss at the hepaticojejunostomy suspicious for stricture (green arrow). Subsequent percutaneous transhepatic cholangiogram showed a high-grade anastomotic stricture that was treated with an internal/external biliary drain.
Total Pancreatectomy
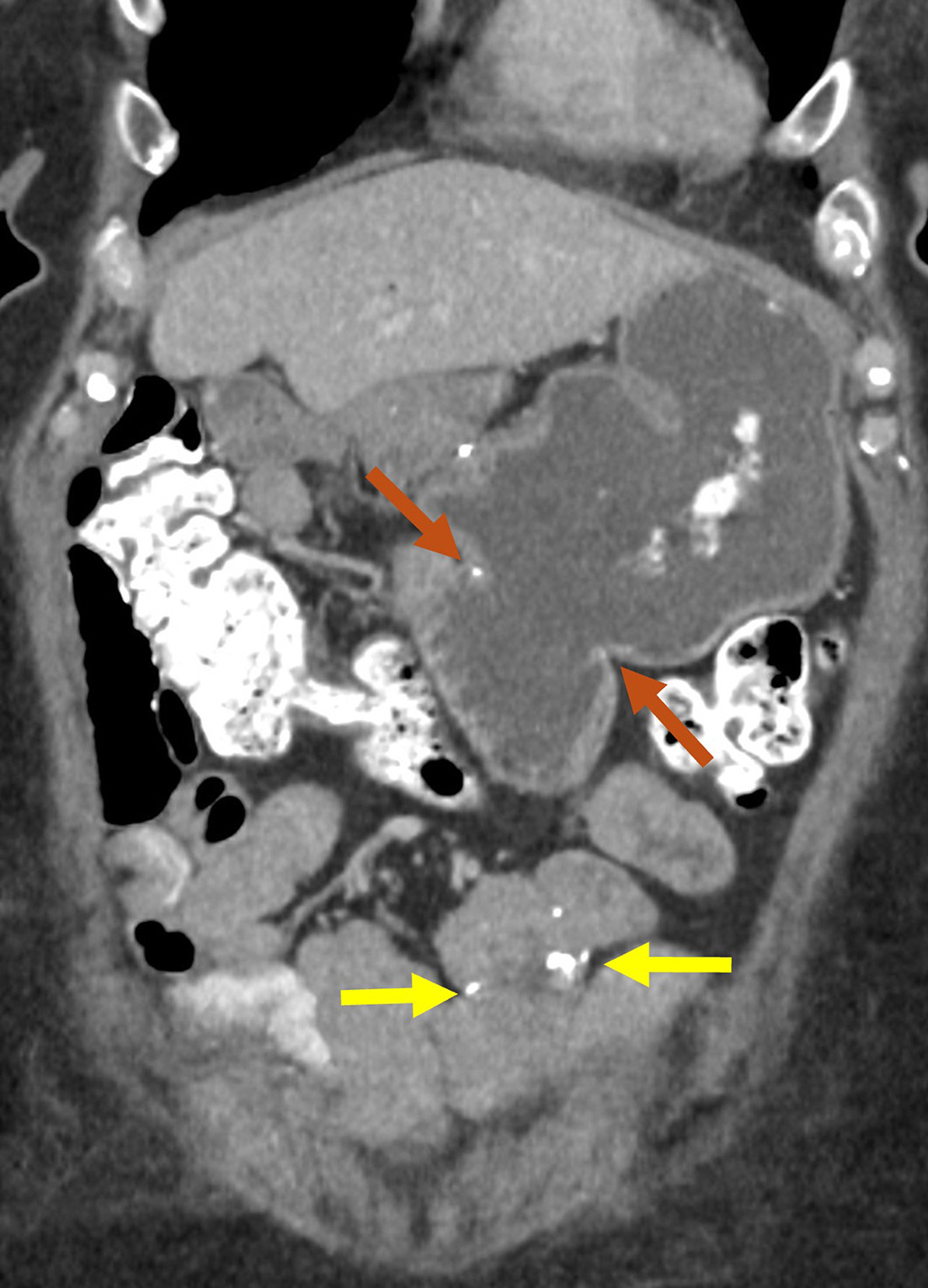
Removal of the entire pancreas is performed for malignancies, multifocal tumors, cystic neoplasms (intraductal papillary mucinous neoplasm), refractory chronic pancreatitis, or in a “staged” or “completion” fashion following an initial partial pancreatectomy. In addition to the pancreas, the spleen, duodenum, gallbladder, and CBD are removed.31 Reconstruction involves hepaticojejunostomy and gastrojejunostomy. Methods for identifying pancreatectomy ( Figure 8 ), and its postsurgical complications, are similar to those described for Whipple, but without the risk of a pancreatic leak. Development of a bile leak or marginal ulcer can require reintervention ( Table 1 ).36
Normal contrast-enhanced coronal CT reformation with oral contrast of a patient with prior total pancreatectomy. The hepaticojejunostomy is sometimes identified by the presence of pneumobilia (white arrow).
Puestow
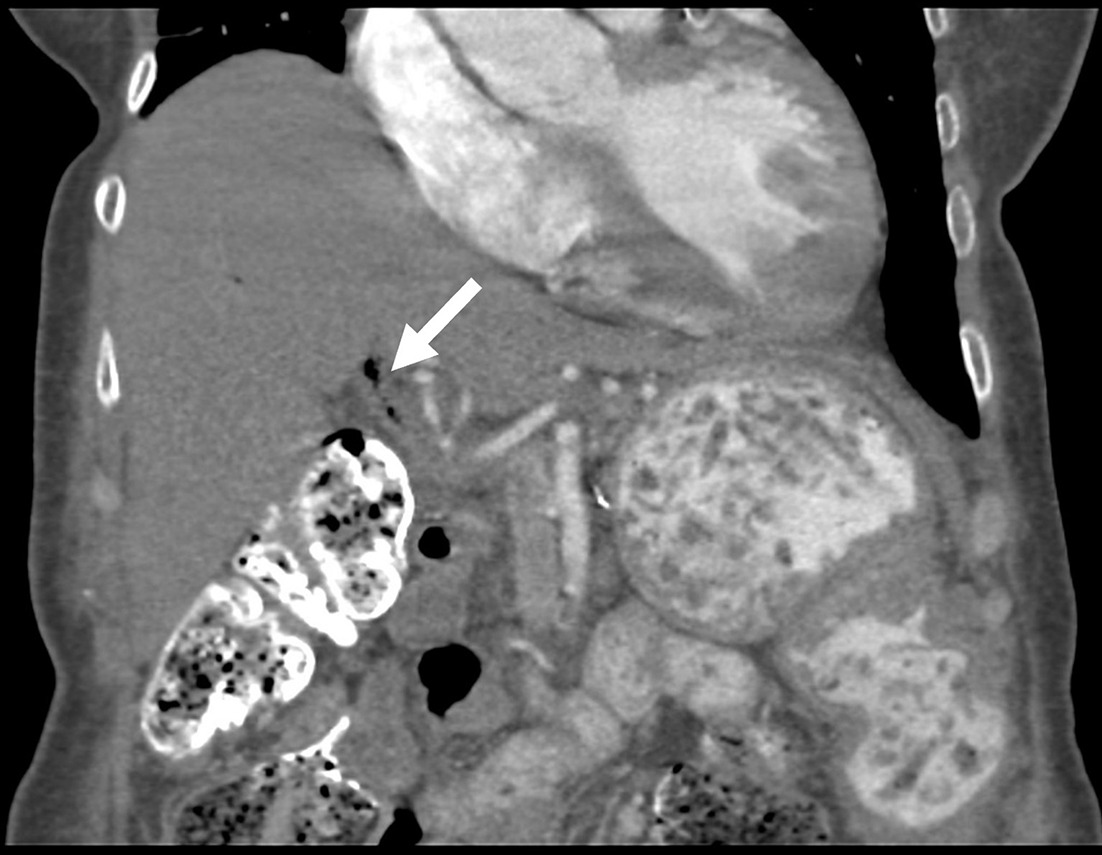
The Puestow procedure, or lateral pancreaticojejunostomy, is performed as a pancreatic duct drainage procedure for chronic pancreatitis. This consists of creating a longitudinal incision along the main pancreatic duct; creation of a jejunal Roux limb and pancreaticojejunostomy along the pancreatic duct incision; and jejunojejunostomy to restore GI tract continuity ( Figure 9 ). There are also variations of this procedure, which involve additional uncovering the pancreatic head (Frey procedure) or removal of the pancreatic head (Beger procedure), both of which rely on Roux limb drainage.37
Contrast-enhanced axial CT demonstrates normal appearance of a Puestow procedure, with the jejunal limb (yellow arrow) and atrophic remnant pancreas (orange arrow).
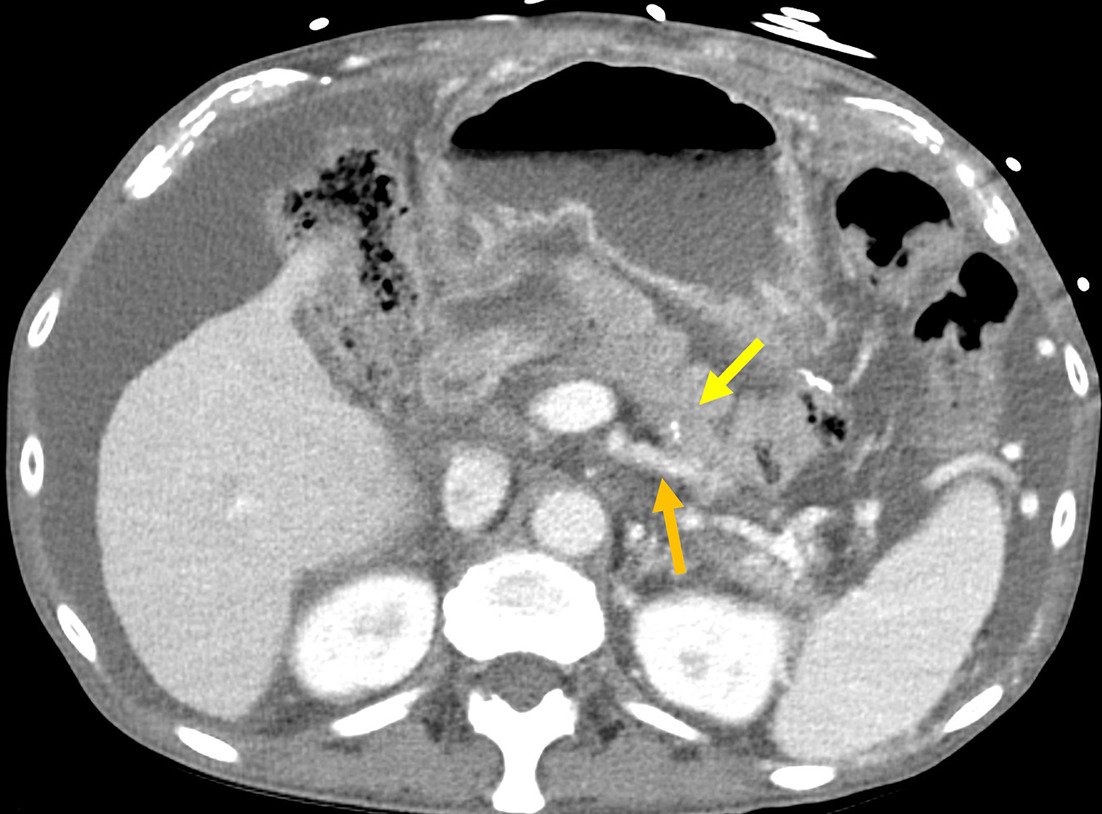
Associated complications include intra-abdominal infection, hemorrhage, obstruction, and rarely anastomotic leak, with infection among the more common ( Table 1 ).38
Roux-en-Y Hepaticojejunostomy
Roux-en-Y hepaticojejunostomy (RYHJ) is a reconstruction technique applied following biliary bypass for benign or post-transplant stricture, resection of malignancy involving the biliary tree, choledochal cyst resection, or repair of iatrogenic or traumatic injury.39 The technique involves the creation of a jejunal Roux limb, hepaticojejunostomy, and jejunojejunostomy to restore GI tract continuity. A variant is the choledochoduodenostomy, which is a technically simpler procedure where the bile duct is directly re-anastomosed to duodenum, avoiding transection and re-anastomosis of jejunum. When feasible, RYHJ, which has fewer long-term complications, is favored.40
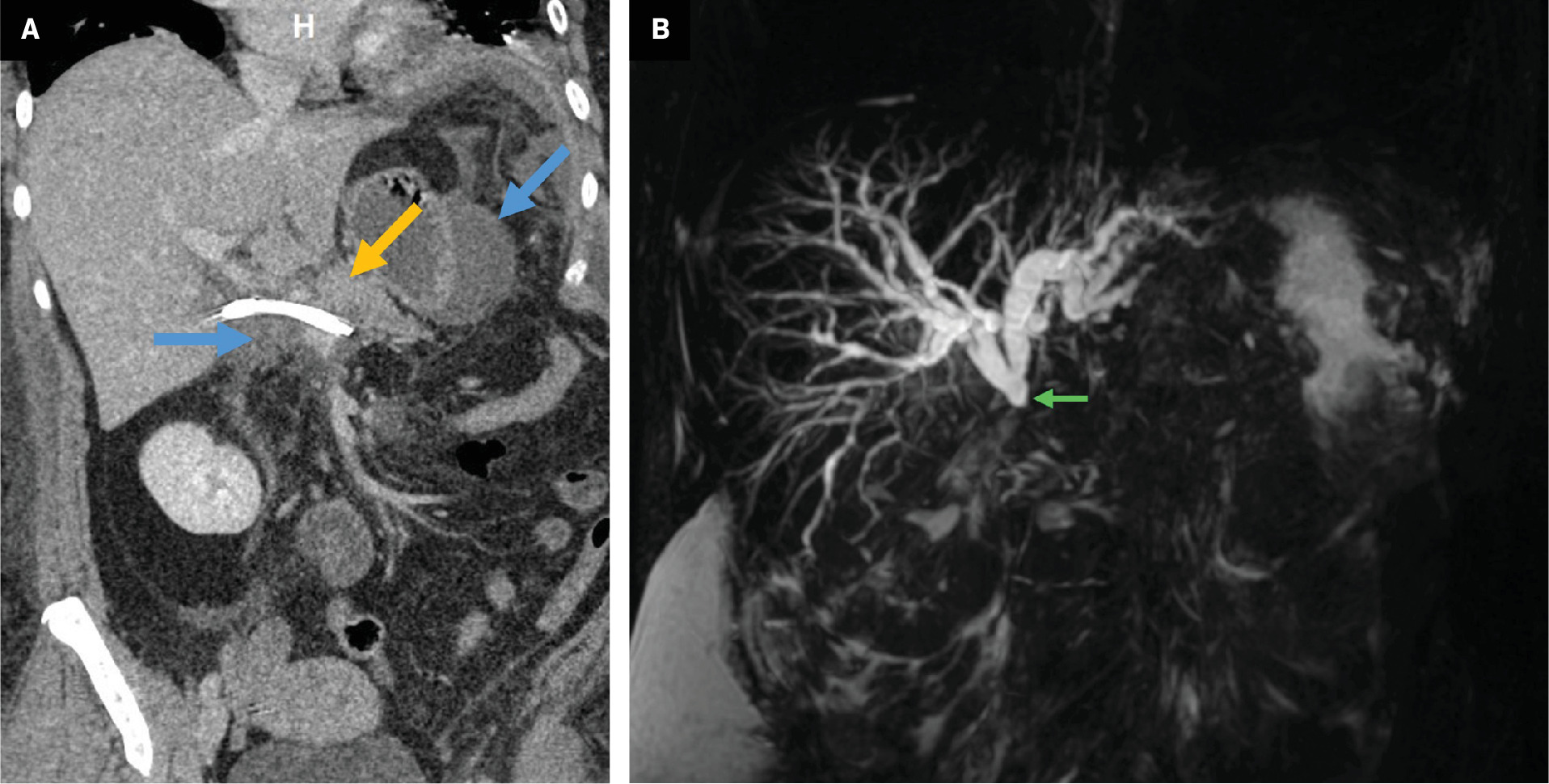
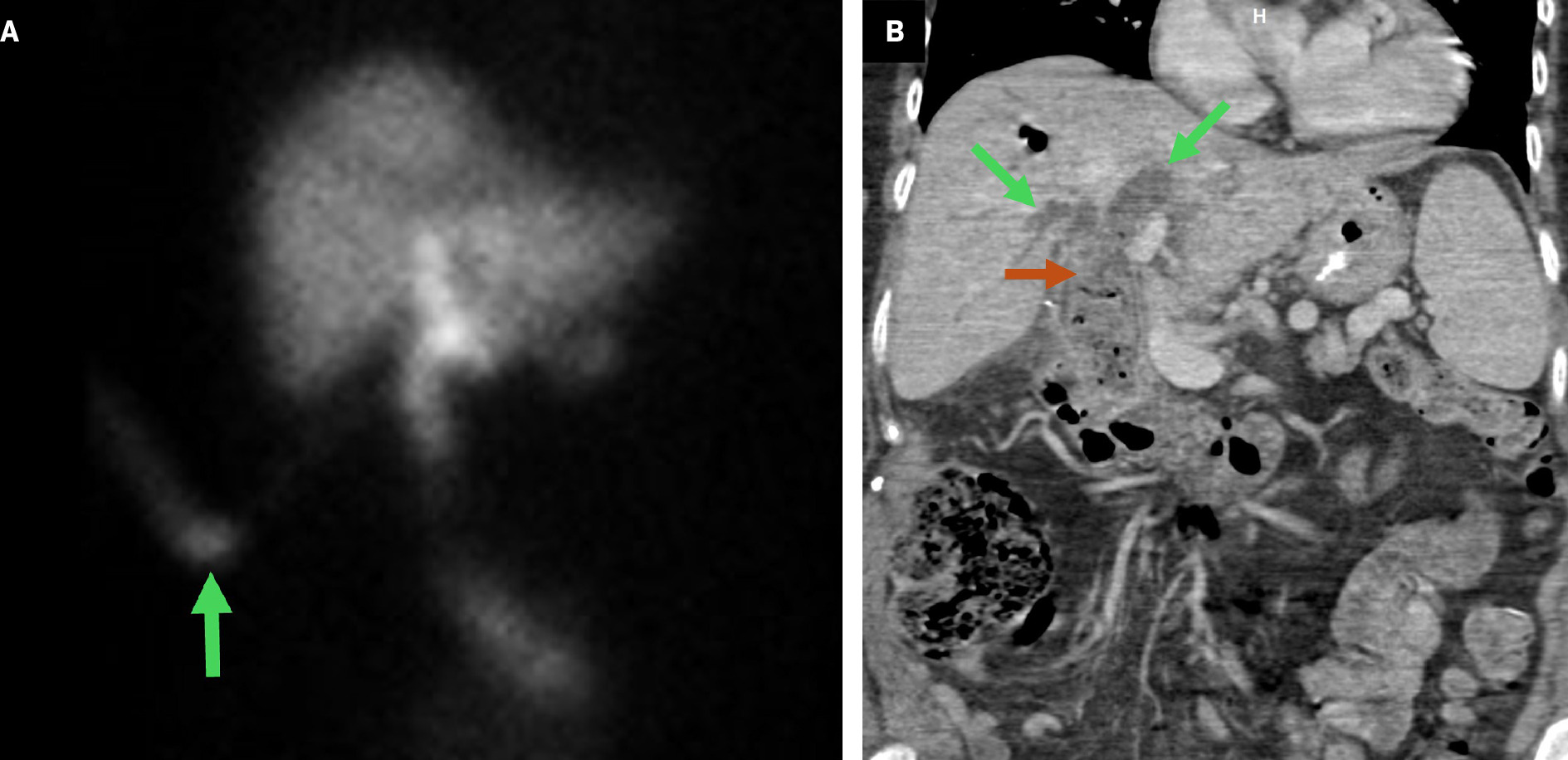
Tips to identify the hepaticojejunostomy are like those previously described. Complications associated with RYHJ are predominantly related to the biliary anastomosis ( Table 1 ).41, 42 Nuclear medicine HIDA scan and MRCP can be useful additional studies for the evaluation of bile leak and stricture ( Figure 10 ). The rare complication of sump syndrome is more prevalent in choledochoduodenostomy and typically occurs in a side-to-side anastomosis where debris accumulating in the bile duct distal to the anastomosis causes obstruction ( Figure 10 ).43 As a result, these patients are prone to developing cholangitis.44
Nuclear medicine hepatobiliary iminodiacetic acid scan ( A ) on postoperative day 3 following a choledochojejunostomy revision for choledocholithiasis demonstrates radiotracer activity within a surgical drain terminating in the gallbladder fossa (green arrow), consistent with postoperative bile leak. Contrast-enhanced coronal CT ( B ) reformation of a patient with prior choledochojejunostomy demonstrates intrahepatic biliary duct dilatation (green arrows) and debris in the distal common bile duct (brown arrow), suspicious for sump syndrome. The patient subsequently developed recurrent episodes of cholangitis and required treatment with multiple endoscopic retrograde cholangiopancreatographies.
Conclusion
There are many UGI tract surgeries that result in altered anatomy and the creation of new limbs/conduits. An understanding of the expected postsurgical appearance is fundamental to identify postoperative complications, many of which stem from the creation of new anastomoses. Multimodality evaluation with CT, fluoroscopy, MRI with MRCP, and HIDA scan can aid in further evaluation of suspicious findings and guide patient management.
References
Citation
Seykora T, Vollmer CM, Adeb M.Untangling the Postoperative Upper GI Tract: Imaging Appearance of Altered Anatomy and Its Complications. Appl Radiol. 2025; (1):5-14.
doi:10.37549/AR-D-24-0058
February 6, 2025