Treatment Brings Hope in Alzheimer Disease: Role of Imaging AI
Images
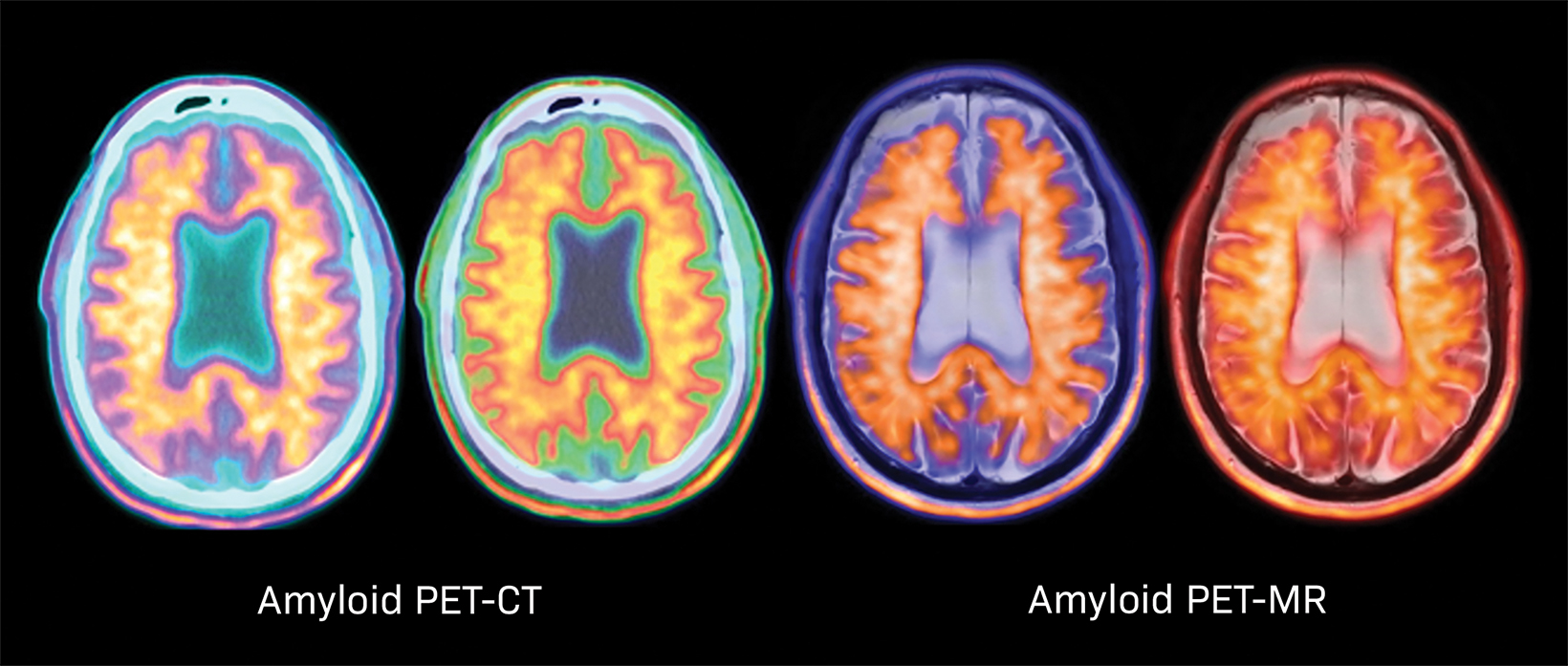


Alzheimer disease (AD) is a devastating, lethal disease affecting 6 million in the US (and 20 million worldwide). The hallmarks of AD in the brain are beta-amyloid (Aβ) plaques, which are imaged with amyloid PET (Figure 1), and neurofibrillary tangles, which are imaged with tau PET.
AD is the most common form of dementia and death rates are rising. For example, while death from heart disease is down 7 percent since 2000, deaths from AD are up 145 percent. The incidence of AD doubles every five years after the age of 60. APOE ε4 status is the strongest genetic risk factor for AD, with 25 percent of the US population carrying one APOE ε4 allele (increasing the risk of AD threefold) and 2-3 percent of the US population carrying two APOE ε4 alleles (increasing risk twelve-fold). One in three seniors will die of dementia, which kills more people than breast and prostate cancer combined.
Artificial intelligence (AI) plays an important role in diagnosis and follow-up of dementia, particularly in the realm of quantitative volumetric neuroimaging (QNI). Quantitative assessment reduces reader subjectivity, and improves interand intrareader consistency, factors referrers value highly. Quantitative analysis includes color-coded segmentation and volumetric assessment of anatomic substructures pertinent to dementia, with comparison to a large normative age and gender-matched database (Figure 2). Values that fall more than two standard deviations from the mean are highlighted. For longitudinal studies, the rate of drop-off from the normative curve can be useful in differentiating mild cognitive impairment (MCI) from progression to AD, as 40 percent of patients with MCI will progress to AD. Assessment of the volumes of pertinent brain substructures can be useful in distinguishing between types of dementia. Some AI products also incorporate clinical information, APOE ε4 status, and additional quantitative imaging features in an attempt to improve prediction of which patients may progress to AD.
Biogen’s Aduhelm™, a monoclonal antibody which targets the epitope on Aβ plaque, and the first potentially disease-modifying therapy for AD, was approved by the FDA under its Accelerated Approval Program (AAP) in June 2021 — a decision seen by some as controversial due to concerns over cost and evidence of efficacy.
While both Biogen’s Phase 3 clinical trials successfully met the surrogate endpoint (effective removal of Aβ plaque as shown on amyloid PET when compared to placebo), only one of the two trials met the primary endpoint of improved cognition. (The high-dose limb of one trial showed a 23 percent improvement.)
The financial impact of untreated AD is high, costing $355 billion in 2021 and rising to $1.1 trillion by 2050 in the US alone. Although Aduhelm remains expensive, Biogen has just slashed the price in half (now 28K/year). While nearly all US states will reimburse on a case-by-case basis, a federal CMS decision to approve payment has yet to be made but is expected no later than April 2022. A nationwide approval isessential to ensure consistent inclusion in hospital formularies and facilitate treatment. While the European Medicine Agency (EMA) recently recommended denying market authorization for Aduhelm, Biogen has formally requested a re-examination. Several other potential disease-modifying therapies are in the overall pharmaceutical pipeline, some of which have already been granted FDA Breakthrough Therapy designation.
Imaging enterprises will be significantly impacted by AD therapy, especially if CMS approves reimbursement. Although not label-required, confirmation of the presence of Aβ plaque is likely to be expected before patients will be treated with Aduhelm. This is accomplished through lumbar puncture, amyloid PET (currently not reimbursed by CMS), or blood tests, such as Precivity AD.
A substantial uptick in the number of MRI studies for AD is expected. A minimum of three brain MRI exams are required for treatment (baseline, and prior to the seventh and tenth monthly infusions, respectively). However, many more brain MRIs will likely be ordered each year, particularly if patients develop any neurologic symptoms or signs during treatment (eg, headache, numbness, weakness) because 41 percent of treated patients will develop amyloid-related imaging abnormalities (ARIA) in the brain.
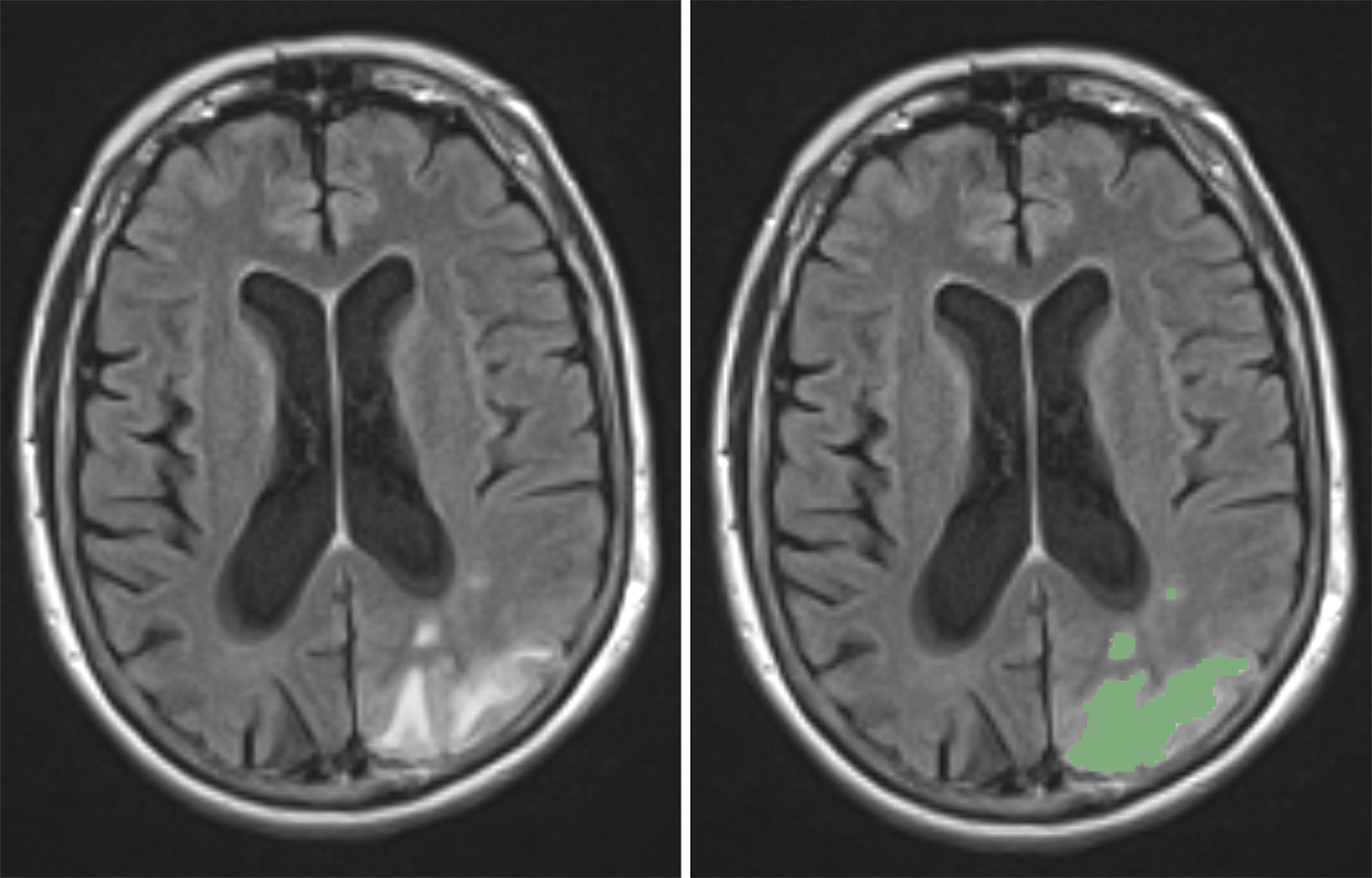
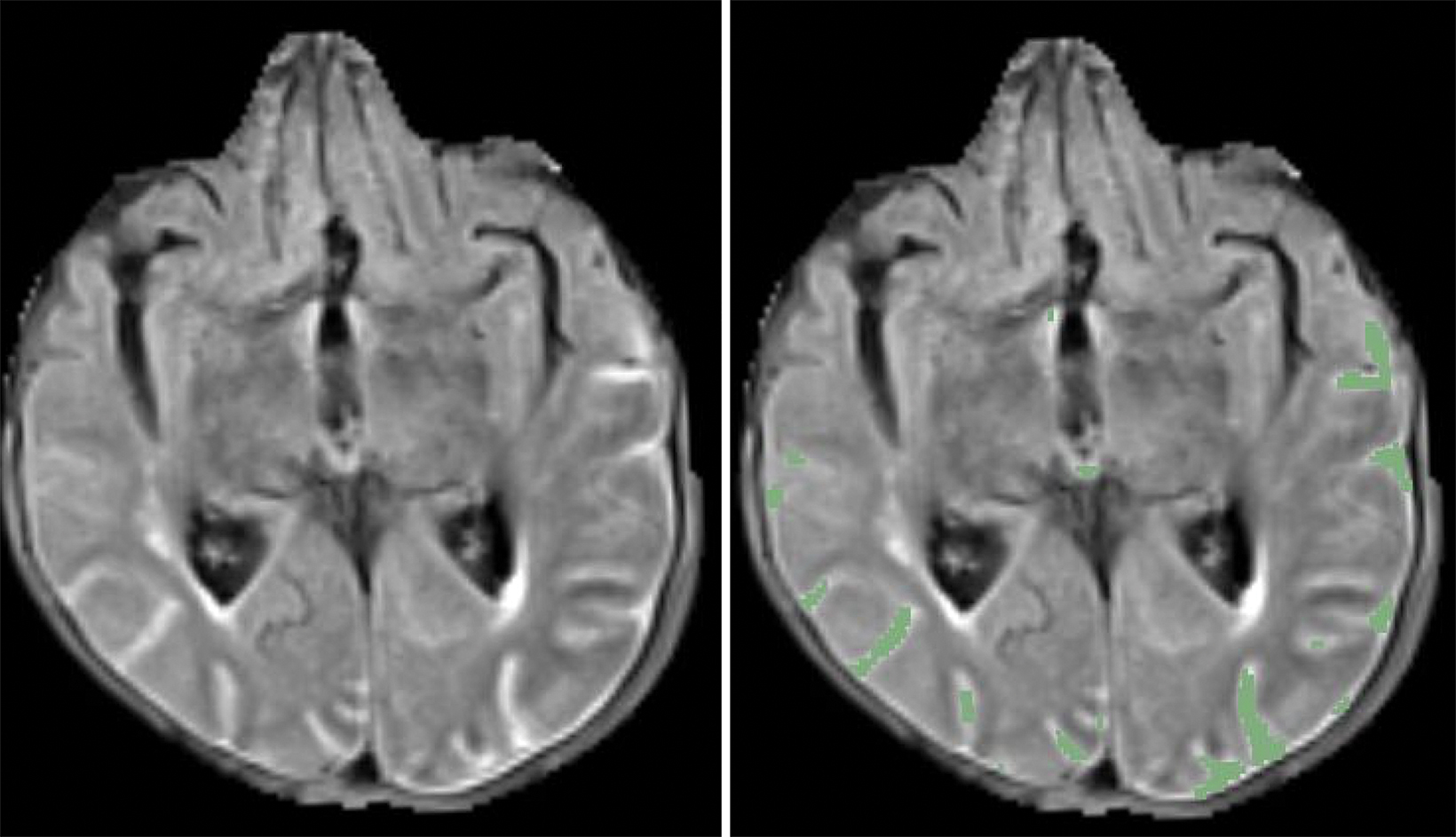
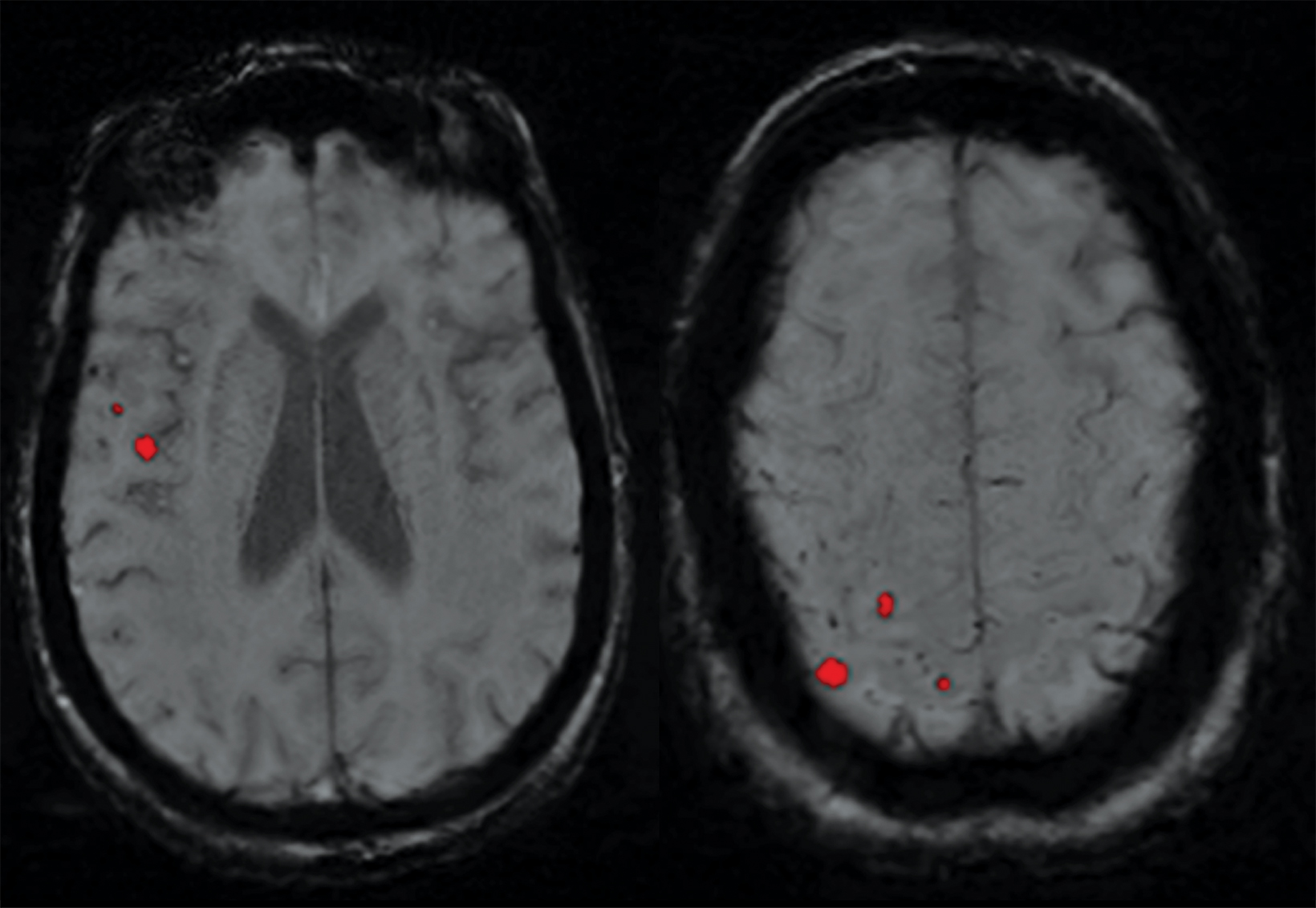
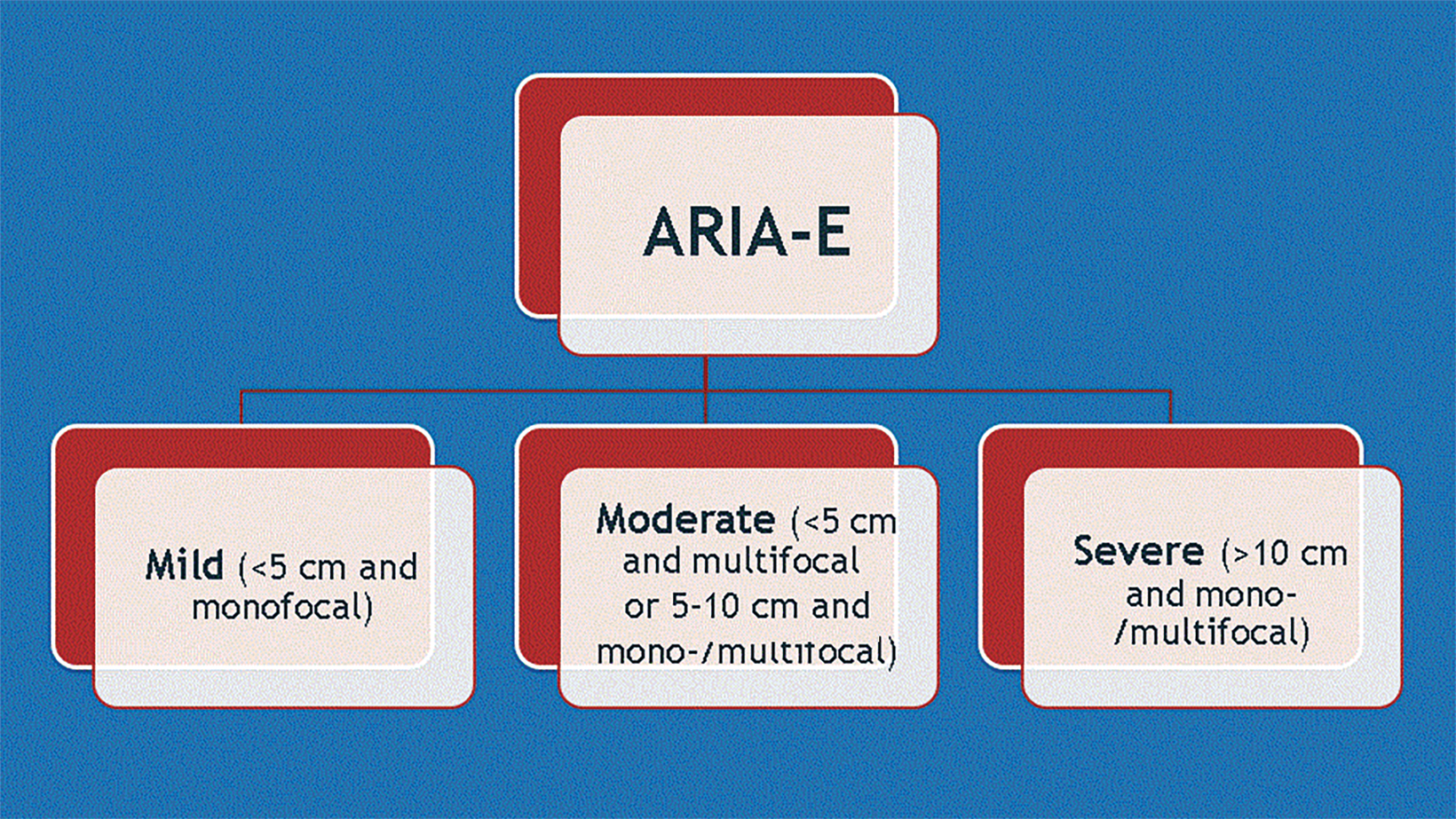
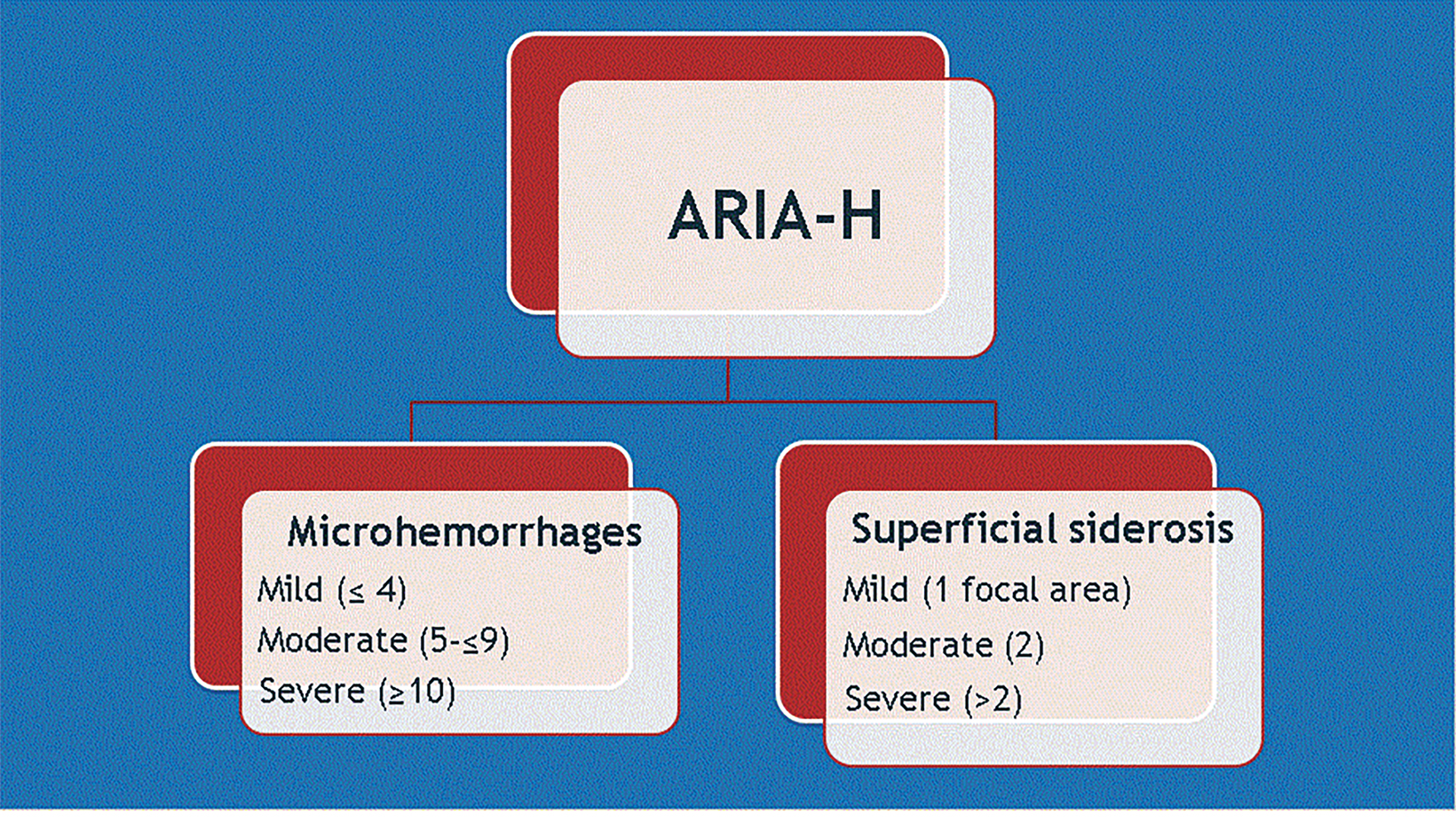
ARIA-E (edema or sulcal effusion) is usually self-limited, even in its severe form, and should not require a medication hold (Figures 3, 4). However, ARIA-H (micro-hemorrhages or superficial siderosis) will require a medication hold if severe (greater than 10 new microhemorrhages or two new superficial sideroses since the prior timepoint), but the hold is only required until follow-up MRI demonstrates stability (Figure 5).
ARIA risk factors include high-dose treatment, baseline cerebral amyloid angiopathy, and APOE ε4 carriers. Fortunately, most (80-90 percent) of patients with ARIA will be asymptomatic. Since the interpreting radiologist is responsible for identifying findings that could result in a medication hold, a global educational initiative is required to educate the radiology community on the appearance of ARIA (Figures 6, 7). A good online resource can be found at Alzimaging.com.
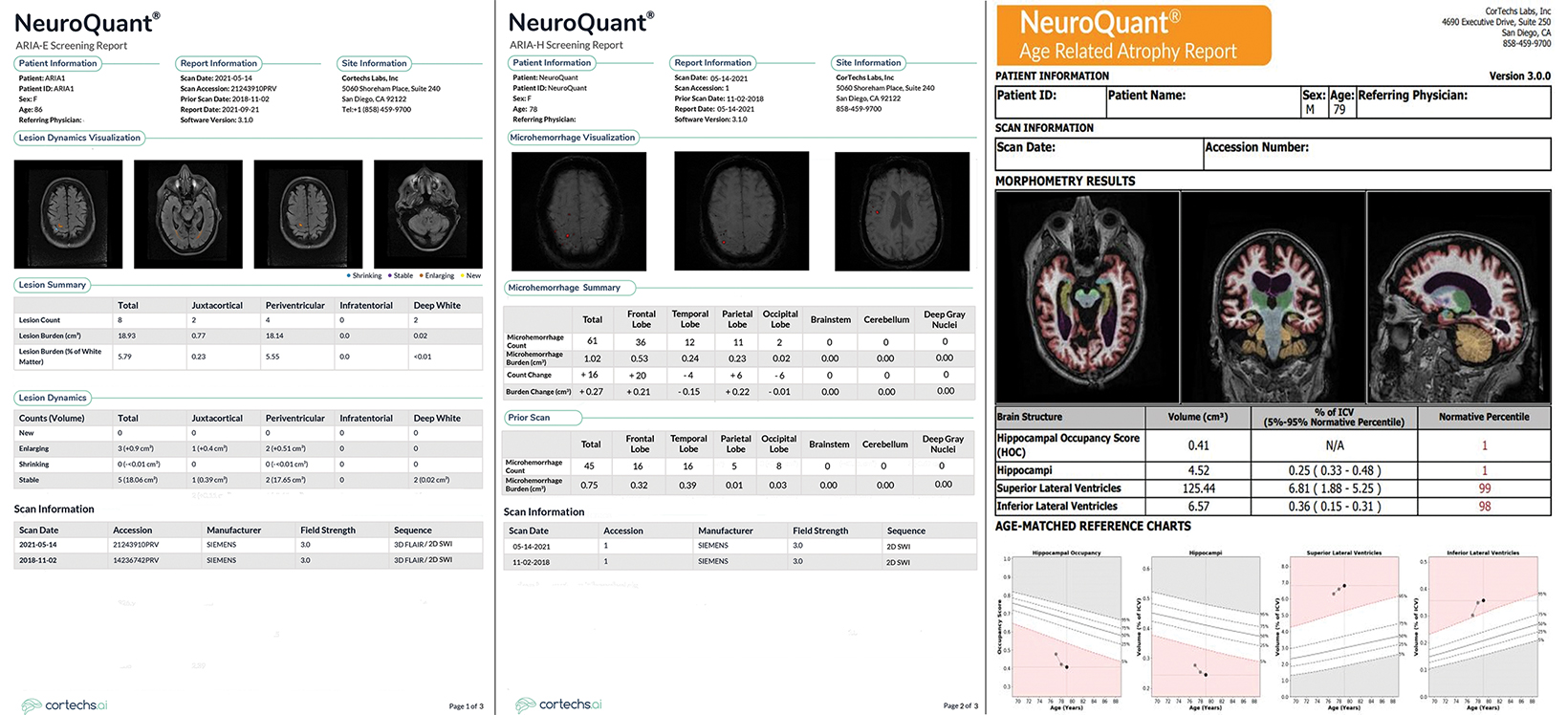
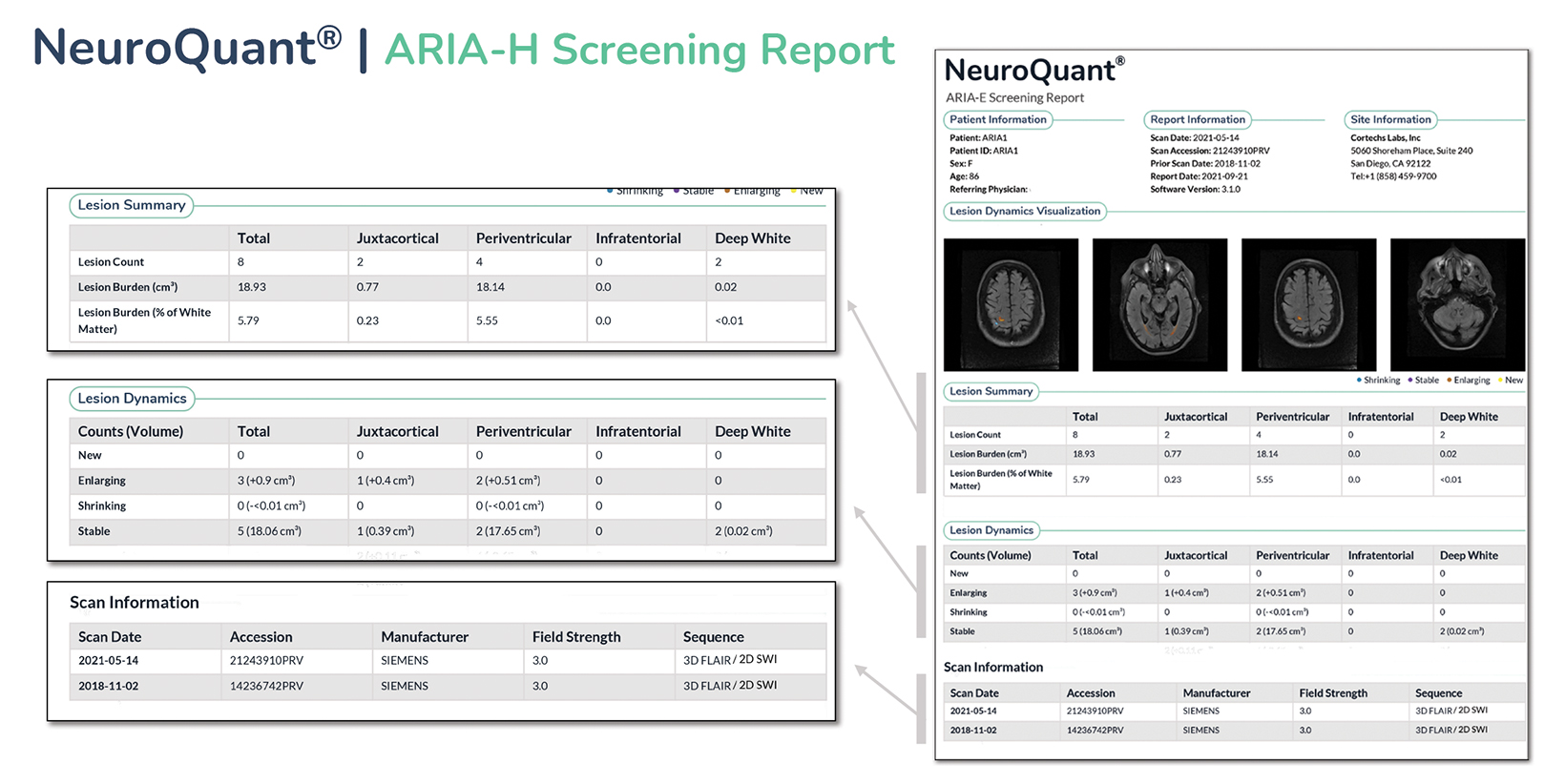
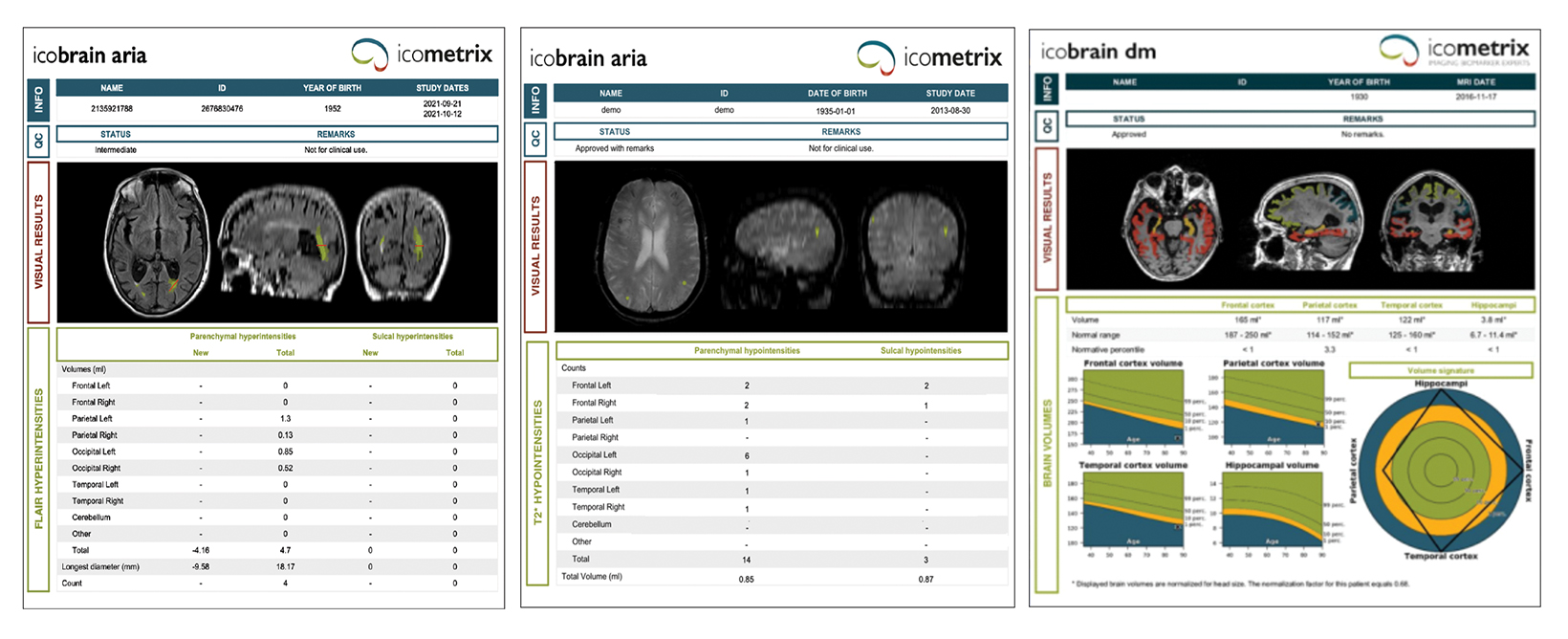
Artificial intelligence has an important and growing role in screening for ARIA. Several QNI AI companies have developed dedicated ARIA functionality based on 3D T1, FLAIR, and blood/hemosiderin sensitive (gradient recalled echo and/or susceptibility weighted imaging) inputs. The reports typically have three components (See Figures 8-11 online). The first is a volumetric calculation of pertinent brain sub-structures (hippocampi and ventricles), which is compared to a normative database to establish a baseline and to help determine if patients demonstrate a hippocampal volume plateau while on treatment. The second consists of ARIA-E screening to assess the volume, count, location, and dynamic change of white matter disease, which is especially useful across longitudinal studies. Finally, the ARIA-H screening component provides the number and location of new microhemorrhages or superficial siderosis along with dynamic change data.
Pharmacology companies are aware of the benefits of AI-fueled QNI and it will be incorporated as subset analysis in Biogen’s upcoming required Phase 4 confirmatory trial, which is expected to start in 2022.
Alzheimer disease is devastating. For the first time, potential disease-modifying therapies are on the horizon, and AI will play an important role in screening for ARIA, as well as in characterization and longitudinal evaluation of patients with dementia.
Citation
S B, LN T.Treatment Brings Hope in Alzheimer Disease: Role of Imaging AI. Appl Radiol. 2022; (1):25-30.
January 6, 2022