Pulmonary Embolism Response Teams: An Integrated Approach to Patient Care
Images

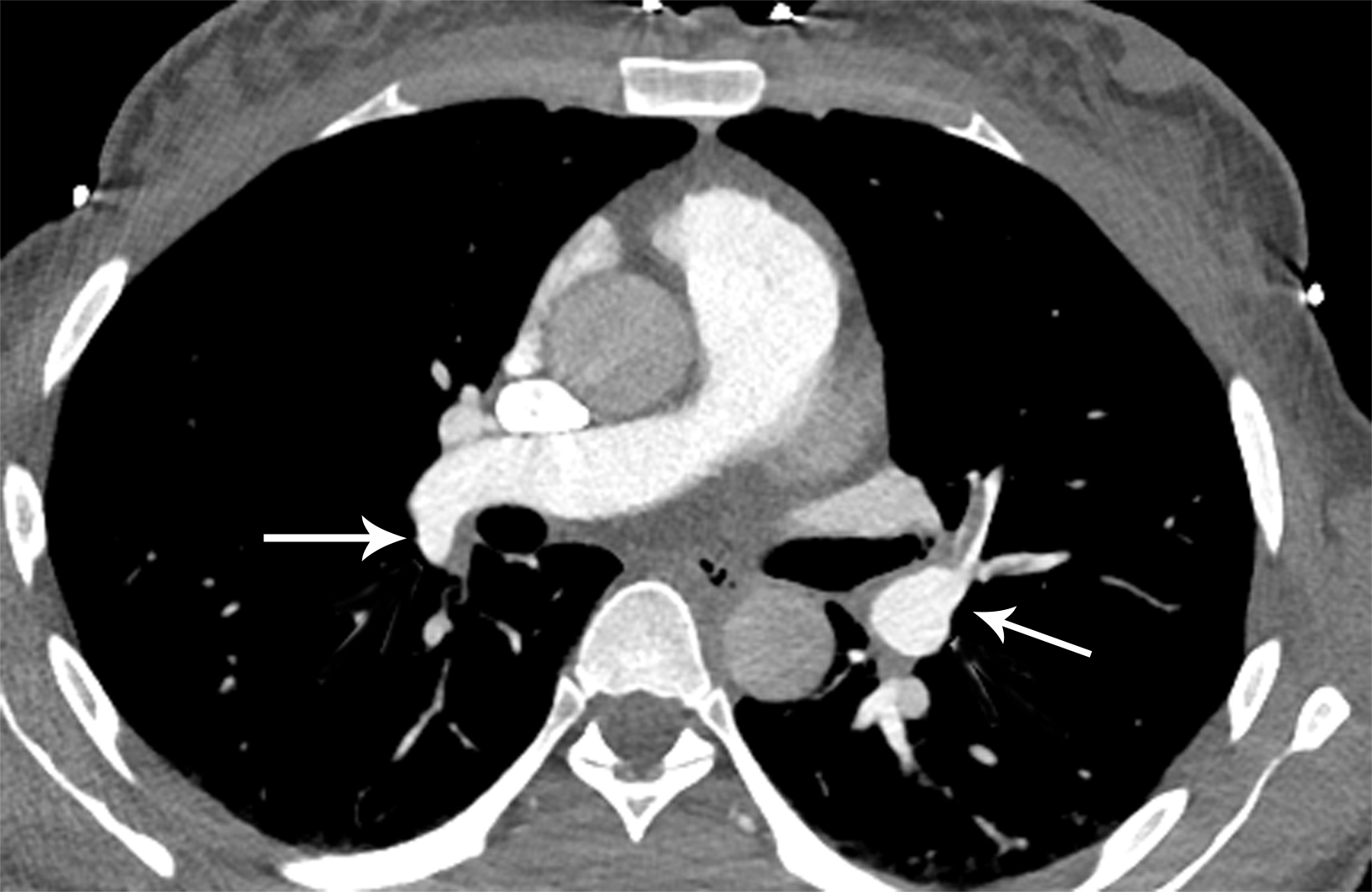
Pulmonary embolism (PE) is the third-leading cause of cardiovascular death in the United States, with an annual mortality of approximately 100,000 people per year.1 While anticoagulation is the primary treatment for acute pulmonary embolism, additional reperfusion strategies exist, including systemic thrombolysis, surgical embolectomy, extracorporeal membrane oxygenation (ECMO) and catheter-directed therapies.
Owing to variations in major professional society recommendations and a lack of data from robust clinical trials, the optimal management for PE remains a topic of debate.2-5 As such, the pulmonary embolism response team (PERT) concept was created in response to rapid advances in therapeutic options and increasing recognition of the complexity involved in the management of patients afflicted by PE.6
The ultimate goal of the PERT is to mobilize rapid medical decision making to improve morbidity and mortality associated with intermediate- and high-risk PE. The goal of this paper is to provide a narrative review of the pulmonary embolism response process, provide an overview of state-of-the-art PE care, and to highlight the critical role of the radiologist (diagnostic and interventional) in PERT.
How the PERT Works
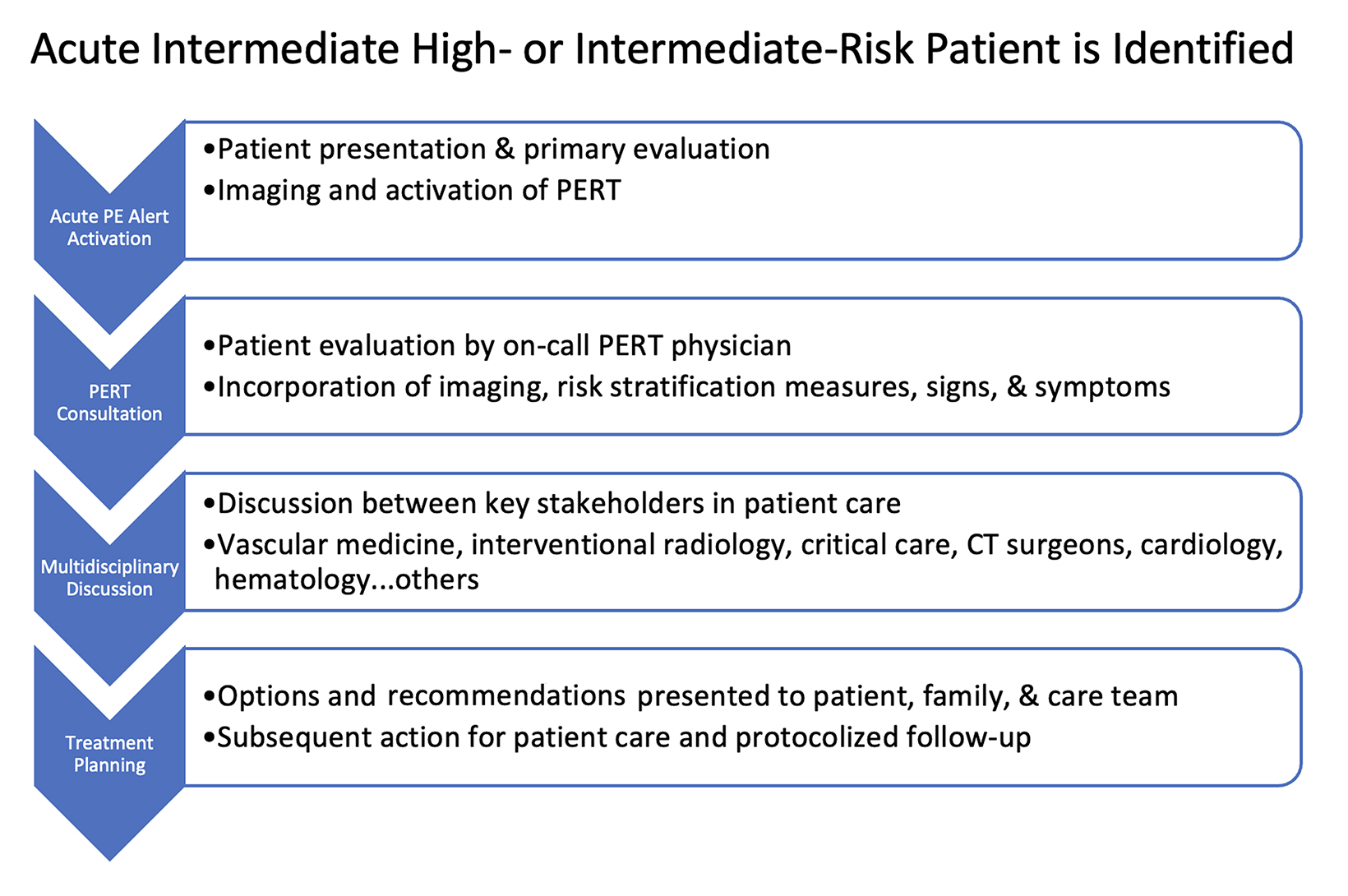
The goal of a PERT is to facilitate rapid, multidisciplinary medical decision making for highly complex and time-sensitive clinical scenarios. The structure of the multidisciplinary PERT varies by institution but can include participants from emergency medicine, pulmonary/critical care medicine, cardiology, vascular medicine, hematology, diagnostic and interventional radiology, vascular surgery, cardiac surgery, and pharmacy. Figure 1 depicts how a PERT activation works at our institution. The PERT system is activated either by calling or paging the PERT on-call member, who then obtains relevant information and coordinates the multidisciplinary discussion. This allows patients with high-risk and select intermediate-risk PEs to receive expedited treatment.
Patient Evaluation
The acute clinical presentation of PE can vary widely. Common signs and symptoms include dyspnea, pleuritic chest pain, tachycardia, presyncope, and hemoptysis. Given their ambiguous nature, risk stratification scoring models such as Wells Criteria, the pulmonary embolism rule-out criteria (PERC) rule, or the Geneva score are used to help derive the pretest probability of a PE in patients presenting in the outpatient emergent setting.7 These scoring models, along with the use of the d-dimer test, establish the need for further radiographic testing.
Imaging and Risk Stratification
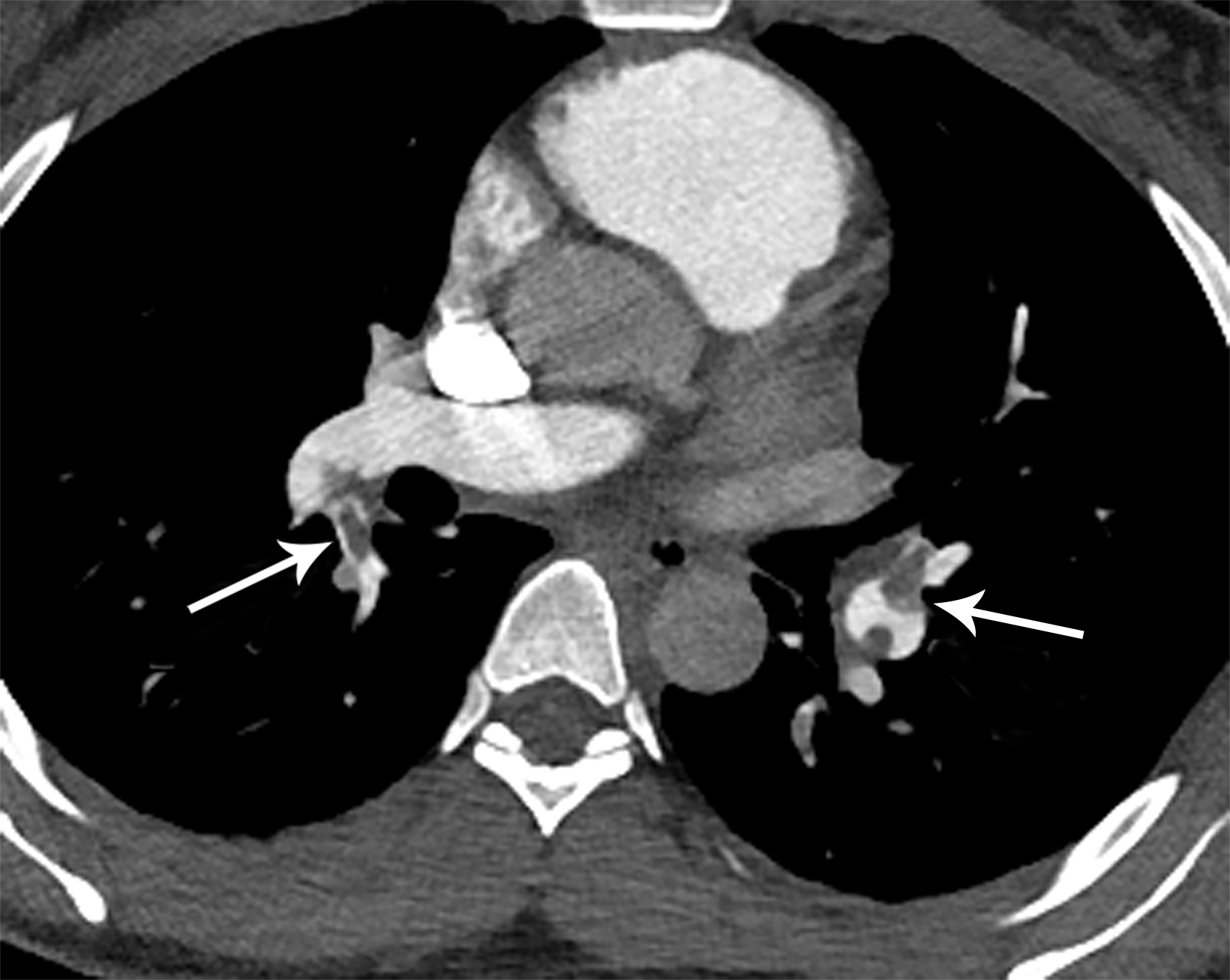
With sensitivity of 83% and specificity of 95% as reported in the PIOPED II study, computed tomography pulmonary angiography (CTPA) is the imaging modality of choice in diagnosing acute PE.8 Findings will include either occlusive or nonocclusive filling defects in the central, lobar, segmental, and/or sub-segmental pulmonary artery branches, depending on the quality of the study. CTPA can determine whether a clot is acute or chronic and oftentimes is able to identify right ventricular (RV) dysfunction (discussed below). Acute clots will often form acute angles with the arterial wall, and the arterial branch may be enlarged compared to patent vessels.9 Additionally, CTPA will also be able to detect alternative diagnoses other than acute PE, if present. In patients with poor renal function or allergies to iodinated contrast, ventilation/perfusion (V/Q) imaging may be performed. Modified PIOPED II criteria specify one of three interpretations: PE present, nondiagnostic, or negative. PE is diagnosed when two or more large, mismatched segmental perfusion defects are present. A normal perfusion scan can exclude PE.8
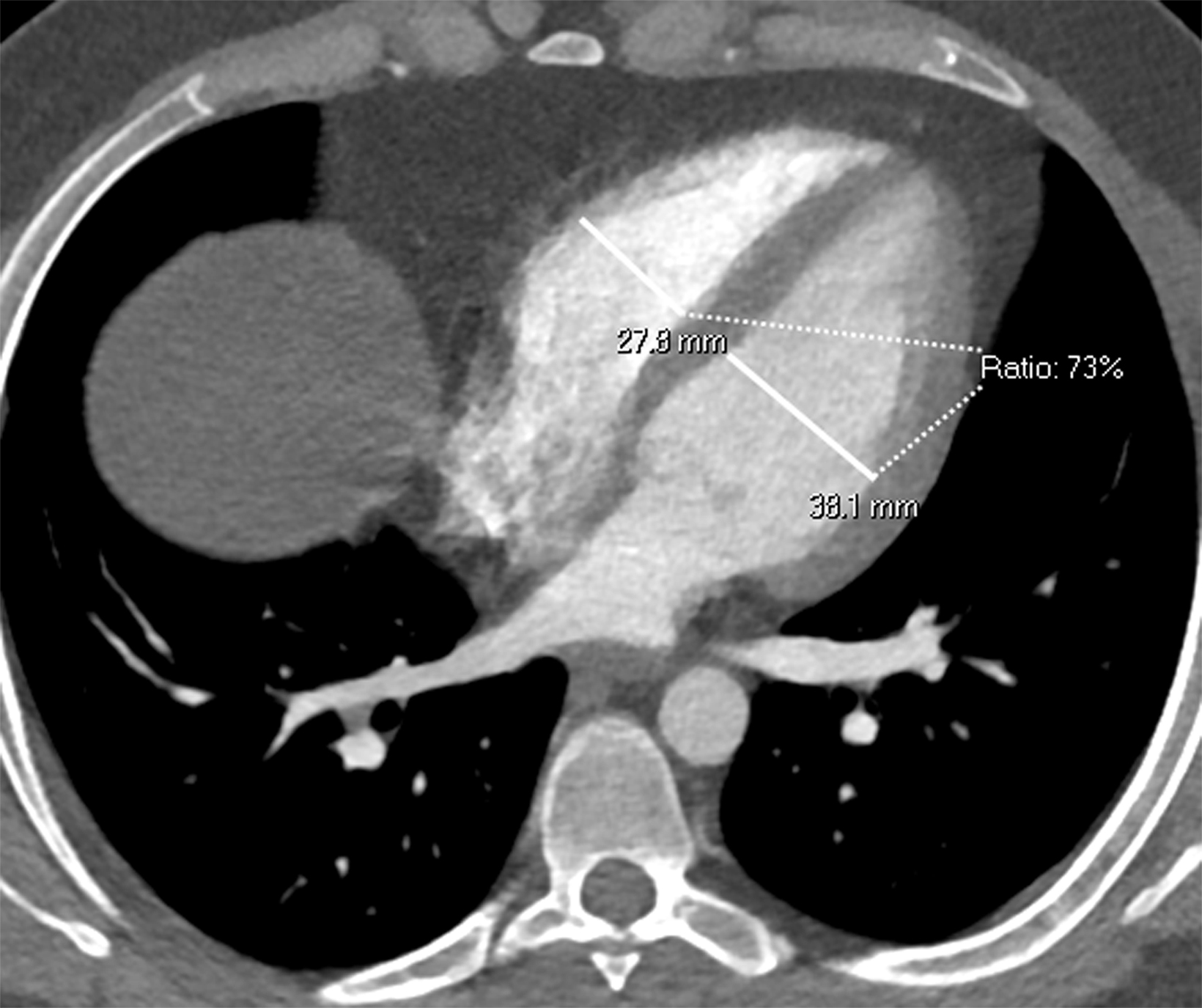
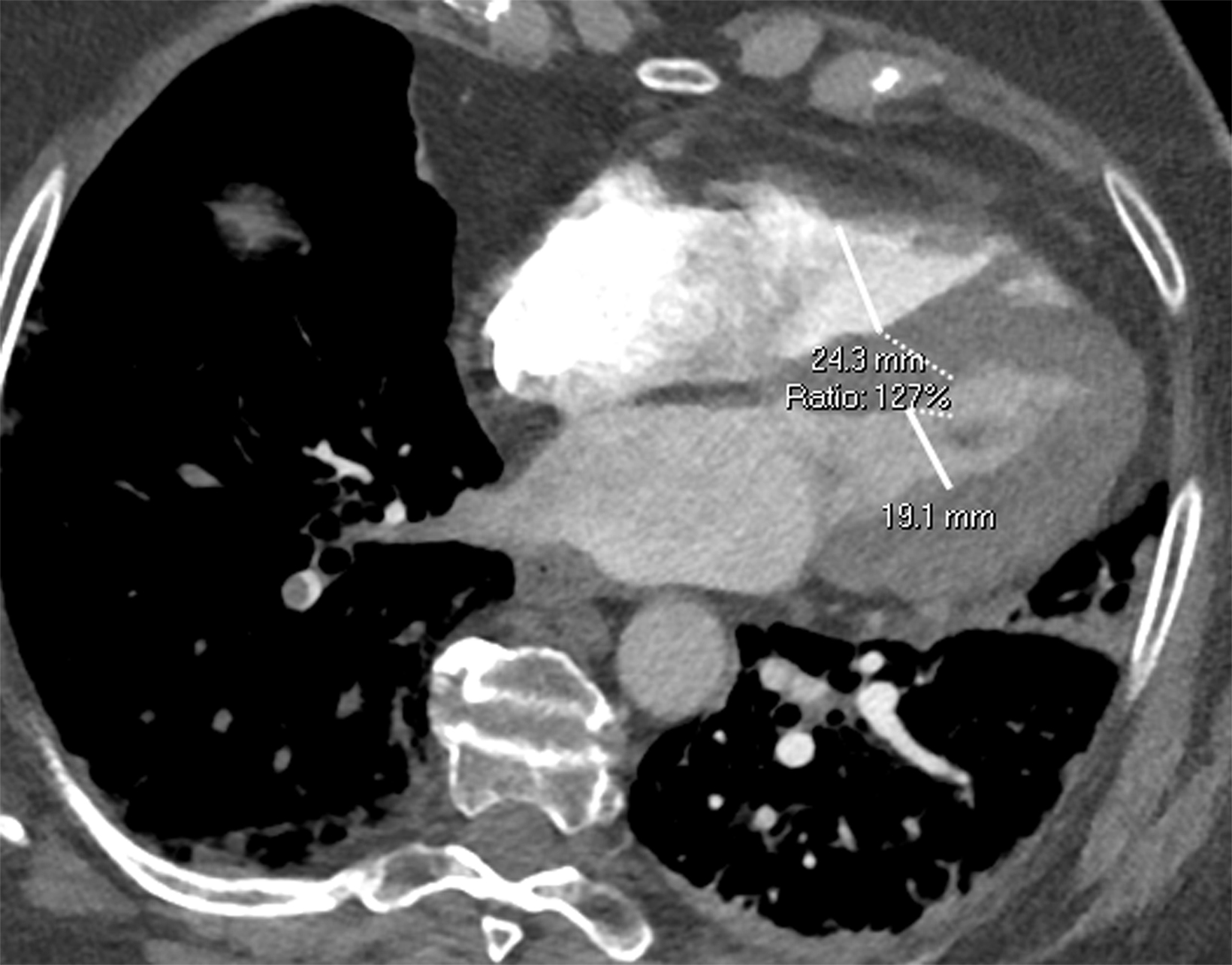
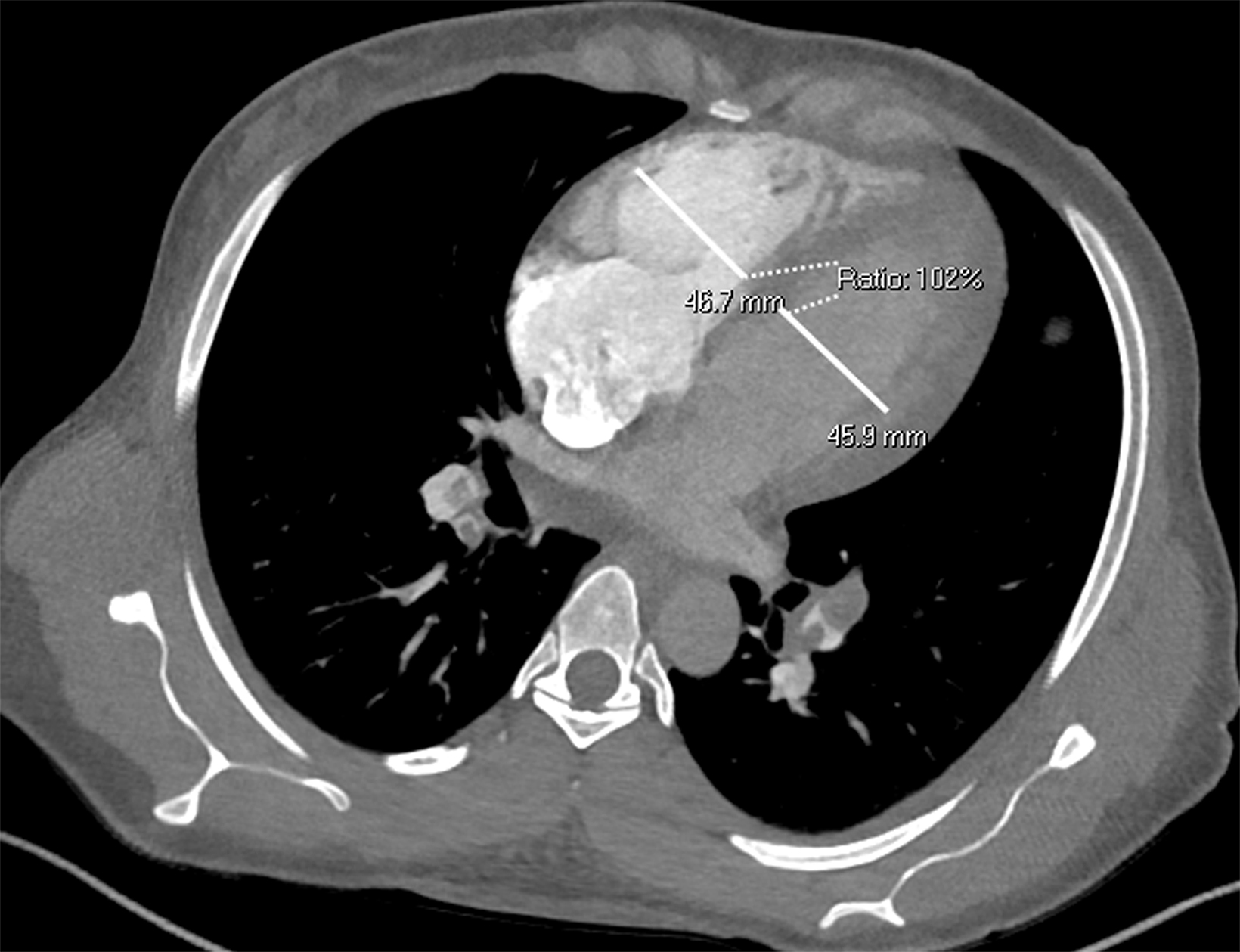
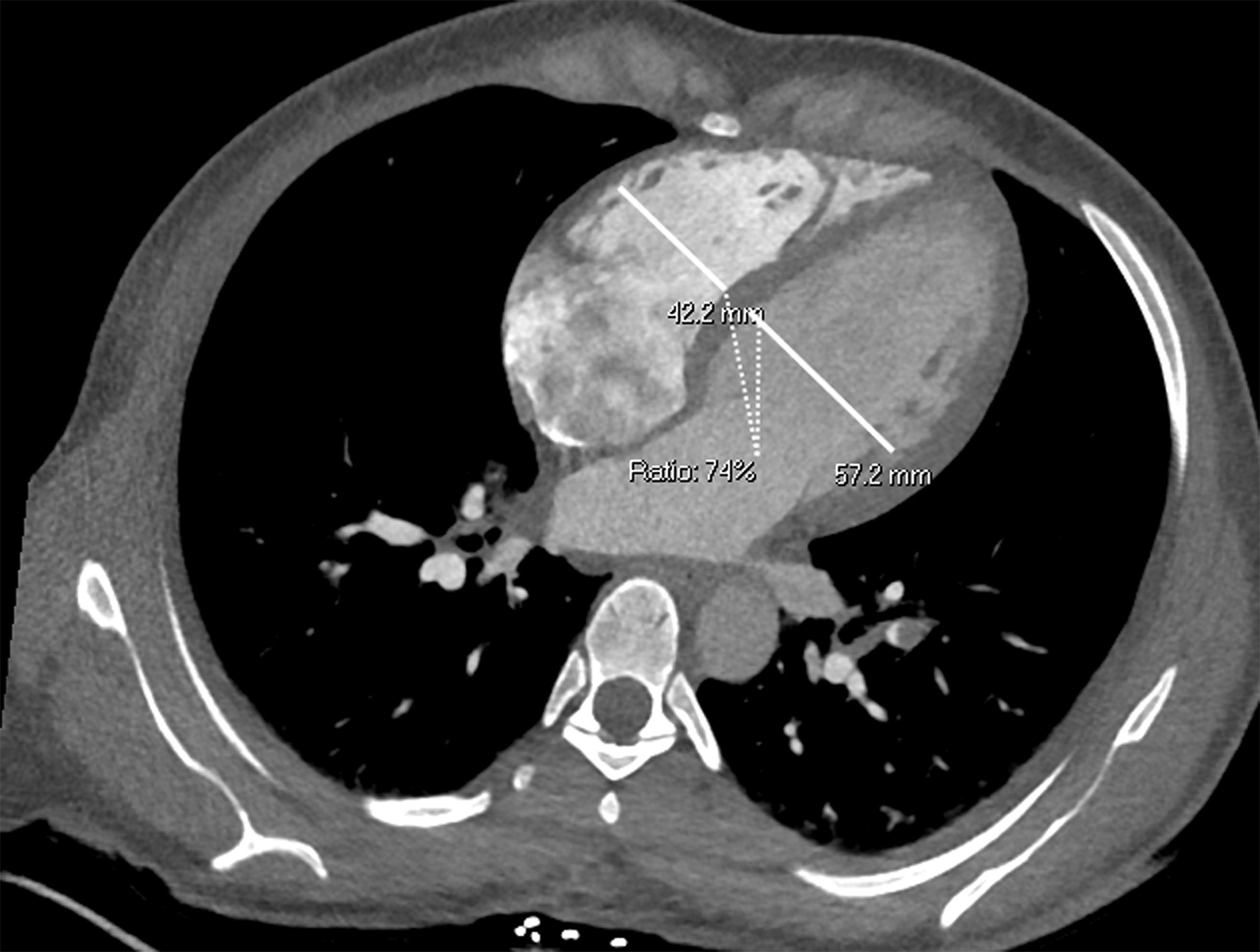
Once a PE is confirmed on imaging, patients are risk-stratified to identify the probability of early mortality and to determine appropriate treatment. Right ventricular failure is the primary cause of short-term death in acute PE. Imaging findings of right heart dysfunction (often denoted “right heart strain”) include flattening or paradoxical bowing of the intraventricular septum, right ventricular enlargement, and contrast reflux into the inferior vena cava and hepatic veins.10 Right ventricular enlargement is
defined by a ratio of RV diameter to LV diameter greater than 0.9.11 The RV/LV ratio can be measured on axial images or multiplanar reconstruction images in the four-chamber axial view. Measurements should be made from endocardial margins, including papillary and trabecular muscles.12 Performed correctly, the different measurement techniques have not shown significant differences in predicting 30-day mortality from acute PE. Figures 2 and 3 depict normal and abnormal RV/LV ratios. However, a CT finding of right heart enlargement has been shown to predict early death (at 30 days) in patients presenting with acute PE.13
Clinical markers of poor RV health, including tachycardia, hypotension, tachypnea, and hypoxemia, are incorporated into clinically validated scoring systems such as the PE Severity Index (PESI) to predict 30-day mortality.14 The European Society of Cardiology (ESC) has used the PESI score in combination with cardiac biomarkers (troponin, B-type natriuretic peptide [BNP], lactate, and creatine) and advanced cardiac imaging (echocardiography) to provide a unified stratification system that offers treatment options.2 According to the ESC model, any patient who exhibits hemodynamic instability is considered “high risk” for early mortality. Hemodynamic instability is defined by cardiac arrest, persistent systolic blood pressure less than 90 mmHg for greater than 15 minutes, use of vasopressors to achieve a systolic blood pressure ≥ 90 mmHg with evidence of end-organ hypoperfusion, or a systolic blood pressure drop ≥ 40 mmHg from the patient’s baseline. High-risk patients are offered hemodynamic support and considered for reperfusion therapies as appropriate.15 Patients who are otherwise hemodynamically stable are stratified into the low-risk or intermediate-risk categories.2
Patient Management
Anticoagulation is the mainstay of acute PE therapy. Initial preference for anticoagulation is highlighted using low-molecular weight heparin (LMWH) or fondaparinux, owing to improved 30-day mortality, decreased risk of hemorrhage, and decreased recurrence of thrombotic events. Unfractionated heparin remains an option in patients with contraindications to LMWH.16,17
Whether a patient receives advanced therapies in addition to anticoagulation depends on their risk stratification. High-risk patients should receive appropriate hemodynamic and respiratory support and be considered for reperfusion therapies such as systemic thrombolysis, catheter-directed treatment, and surgical embolectomy.
Systemic thrombolysis involves the administration of recombinant tissue-type plasminogen activator (rtPA) to improve pulmonary artery obstruction, pulmonary artery pressure, and pulmonary vascular resistance. rtPA use in high-risk PE patients is associated with improved mortality.18,19 Absolute contraindications to systemic thrombolysis include history of hemorrhagic stroke, recent ischemic stroke, intracranial neoplasm, recent major trauma, and active bleeding. Relative contraindications to systemic lysis include hypertension (systolic BP > 180 mmHg), recent non-intracranial bleeding, recent surgery/invasive procedures, ischemic stroke > 3 months previous, or age > 75.
Appropriate hemodynamic and respiratory support can include high-flow oxygen, mechanical ventilation, vasopressors, inotropes, and mechanical circulatory support. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is helpful in patients with circulatory collapse and/or cardiac arrest with or without additional therapies.20 However, data from randomized controlled trials is lacking to support the efficacy and safety of general ECMO use.15,21
A consensus statement from the PERT Consortium suggests surgical embolectomy in high-risk patients with contraindications to, or failure of, systemic or catheter directed thrombolysis or thrombectomy. A similar recommendation is suggested for intermediate-risk patients with significant comorbidities that could lead to clinical deterioration.22 Right-heart thrombi and thrombus-in-transit are other scenarios where surgical embolectomy may be considered as first-line therapy.23 Perioperative mortality in the past could be as high as 11%, but with improved patient selection and surgical techniques, mortality has fallen significantly.
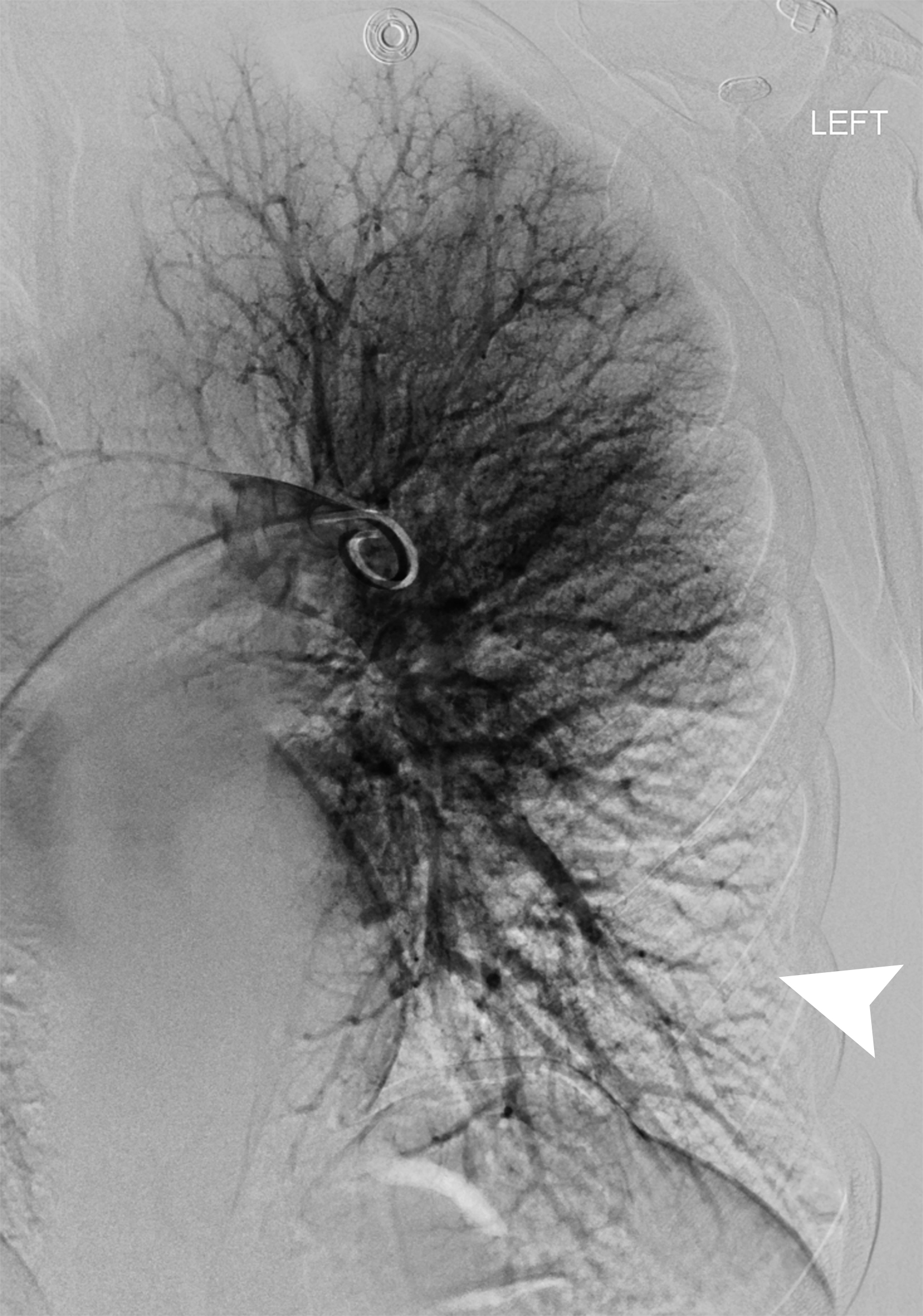
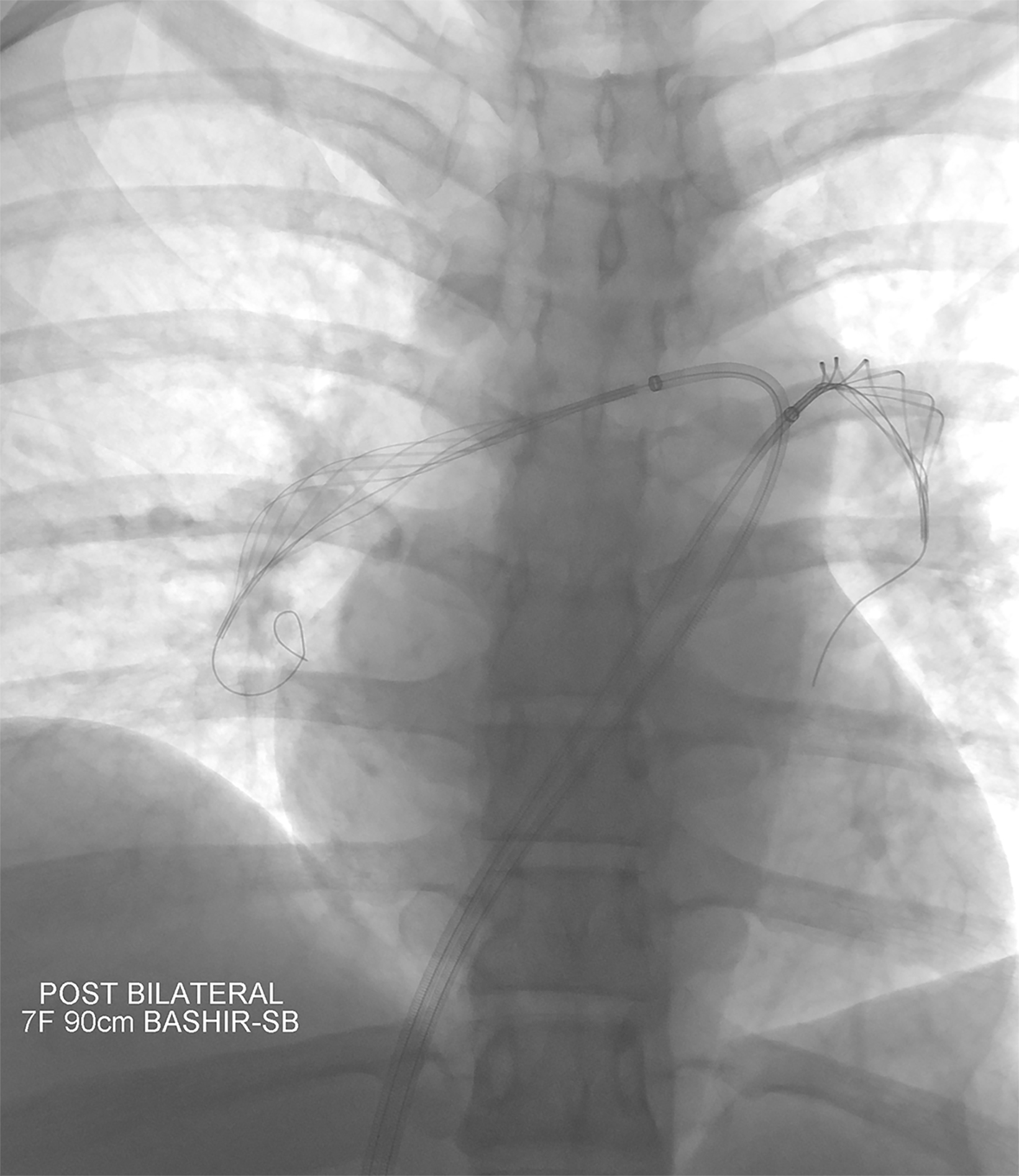
The goals of interventional therapies in patients considered intermediate risk or high risk for early mortality are to avoid hemodynamic collapse and expedite symptom resolution. The risks and benefits of thrombolysis are more closely considered in intermediate-risk PE and counterbalanced by untoward outcomes. A variety of endovascular methods can be used to treat acute PE; they include catheter-directed thrombolysis, aspiration and mechanical thrombectomy, and a combination of interventions.
Several single-arm studies of specific devices have shown a reduction in RV/LV ratio at 24 and 48 hours, which is considered a surrogate endpoint.24-28 High-quality randomized data showing a reduction in mortality and progression of disease to chronic thromboembolic disease is lacking.
An early randomized study of CDT versus unfractionated heparin (n=59) found a significant reduction in RV/LV ratio at 24 hours in the CDT group. Ninety-day RV/LV ratio was also better in the CDT group, although not significant.29 A meta-analysis of ultrasound-assisted CDT for PE found a reduction in pulmonary artery systolic pressure, RV/LV ratio, and an improvement in the cardiac index in over 2,000 patients.25 Multidisciplinary PERT teams are helpful in weighing the risks and benefits of each treatment.
The Radiologist’s Role on the PERT
Diagnostic radiologists are truly the gatekeepers of advanced PE interventions and other treatment modalities. Their appropriate and prompt identification of acute PE and additional parameters such as right-heart strain can lead to the administration of all appropriate interventions in a timely manner, as discussed in the “Risk Stratification” section. We have found that consistently including the RV/ LV ratio on CTPA and writing “consider paging PERT” on the report is very helpful for prompt treatment plan discussion by the PERT.30 Given the advances in and increased use of endovascular catheter-based interventions, the role of interventional specialists is also paramount in managing patients with PE.
Conclusion
Historically, the management of patients with acute pulmonary embolism, especially those at intermediate-high risk, has been haphazard, resulting in delay of potentially life-saving PERT programs have been shown to improve survival and other clinically relevant outcomes.30-33 To achieve improved outcomes, a team-based approach involving clinicians from the initial patient encounter (emergency physicians), consultants (vascular medicine, hematologists, and critical care physicians), diagnostic and interventionalist radiologists, and cardiothoracic surgeons, is required at times. Developing these programs can streamline patient care and result in better outcomes in PE patients at high risk for early mortality.
References
- Wendelboe AM, Raskob GE. Global burden of thrombosis. Circ Res.2016;118(9):1340-1347.
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. 2020;41(4):543-603.
- Giri J, Sista AK, Weinberg I, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: A scientific statement from the American Heart Association. Circulation. 2019;140(20):e774-e801.
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830.
- Stevens SM, Woller SC, Baumann Kreuziger L, et al. Executive Summary: Antithrombotic therapy for VTE disease: Second update of the CHEST guideline and expert panel report. Chest. 2021;160(6):2247-2259.
- Provias T, Dudzinski DM, Jaff MR, et al. The Massachusetts General Hospital Pulmonary Embolism Response Team (MGH PERT): creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hosp Pract 1995. 2014;42(1):31-37.
- Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711.
- ostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology. 2008;246(3):941-946.
- Wittram C, Maher MM, Yoo AJ, Kalra MK, Shepard JA, McLoud TC. CT angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. Radiographics. 2004;24(5):1219-1238.
- Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354(22):2317-2327.
- Sista AK, Kuo WT, Schiebler M, Madoff DC. Stratification, imaging, and management of acute massive and submassive pulmonary embolism. Radiology. 2017;284(1):5-24.
- Lu MT, Demehri S, Cai T, et al. Axial and reformatted four-chamber right ventricle-to-left ventricle diameter ratios on pulmonary CT angiography as predictors of death after acute pulmonary embolism. AJR Am J Roentgenol. 2012;198(6):1353-1360.
- Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110(20):3276-3280.
- Dudzinski DM, Giri J, Rosenfield K. Interventional treatment of pulmonary embolism. Circ Cardiovasc Interv. 2017;10(2):e004345.
- Elbadawi A, Mentias A, Elgendy IY, et al. National trends and outcomes for extra-corporeal membrane oxygenation use in high-risk pulmonary embolism. Vasc Med. 2019;24(3):230-233.
- Robertson L, Jones L. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for the initial treatment of venous thromboembolism. Cochrane Database Syst Rev. 2017.;2(2):CD001100. doi: 10.1002/14651858.CD001100.pub4.
- Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6):1382-1390.
- Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015;36(10):605-614.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost JTH. 2014;12(4):459-468.
- Strobel R, Khaja M, Peruri A, et al. Veno-arterial extracorporeal membrane oxygenation and thrombectomy for massive pulmonary embolism. Heart Surg Forum. 2022;25(2):E241-E242.
- Kaso ER, Pan JA, Salerno M, et al. Venoarterial extracorporeal membrane oxygenation for acute massive pulmonary embolism: A meta-analysis and call to action. J Cardiovasc Transl Res. 2022;15(2):258-267.
- Rivera-Lebron B, McDaniel M, Ahrar K, et al. Diagnosis, treatment and follow up of acute pulmonary embolism: Consensus practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019;25:1076029619853037.
- Keeling WB, Sundt T, Leacche M, et al. Outcomes after surgical pulmonary embolectomy for acute pulmonary embolus: a multi-institutional study. Ann Thorac Surg. 2016;102(5):1498-1502.
- Sista AK, Horowitz JM, Tapson VF, et al. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. JACC Cardiovasc Interv. 2021;14(3):319-329.
- Pei DT, Liu J, Yaqoob M, et al. Meta-analysis of catheter directed ultrasound-assisted thrombolysis in pulmonary embolism. Am J Cardiol. 2019;124(9):1470-1477.
- Tu T, Toma C, Tapson VF, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: The FLARE study. JACC Cardiovasc Interv. 2019;12(9):859-869.
- iazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: The SEATTLE II study. JACC Cardiovasc Interv. 2015;8(10):1382-1392.
- Aggarwal V, Giri J, Nallamothu BK. Catheter-based therapies in acute pulmonary embolism: the good, the bad, and the ugly. Circ Cardiovasc Interv. 2020;13(6):e009353.
- Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479-486.
- Myc LA, Solanki JN, Barros AJ, et al. Adoption of a dedicated multidisciplinary team is associated with improved survival in acute pulmonary embolism. Respir Res. 2020;21(1):159.
- Wright C, Elbadawi A, Chen YL, et al. The impact of a pulmonary embolism response team on the efficiency of patient care in the emergency department. J Thromb Thrombolysis. 2019;48(2):331-335.
- Wright C, Goldenberg I, Schleede S, et al. Effect of a multidisciplinary pulmonary embolism response team on patient mortality. Am J Cardiol. 2021;161:102-107.
- Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150(2):384-393.
References
Citation
ME B, S G, AD M, L M, AM S, Khaja.Pulmonary Embolism Response Teams: An Integrated Approach to Patient Care. Appl Radiol. 2022; (4):14-20.
July 14, 2022