Pituitary metastasis from lung carcinoma presenting as a pituitary adenoma
Images

CASE SUMMARY
A 59-year-old female with no significant medical history presented with a two-month history of progressive peripheral vision loss. Physical examination demonstrated bitemporal hemianopsia; the neurological examination was otherwise unremarkable. Laboratory testing including prolactin levels was unremarkable.
IMAGING FINDINGS
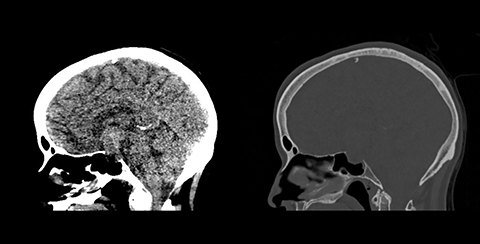
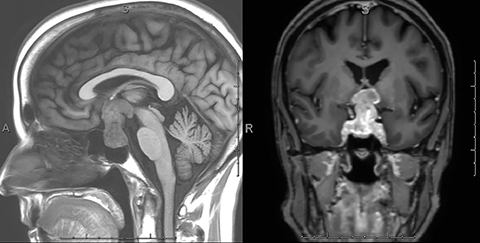
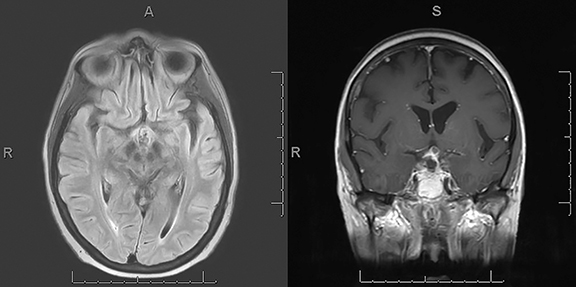
A CT scan of the head demonstrated a large mixed-density sellar/suprasellar mass with expansion of the sella turcica (Figure 1) and erosion of the dorsum sellae. An MRI scan of the brain and sella demonstrated a heterogeneously enhancing T1 iso-intense, T2 mixed-intensity sellar mass with suprasellar extension measuring 3.8 × 2.3 × 2.2 cm (Figures 2,3). The mass compressed the optic chiasm, floor of the third ventricle, and hypothalamus. Additionally, there was extensive T2/FLAIR hyperintensity extending into both optic tracts. The working diagnosis at that time was of a pituitary macroadenoma.
The patient underwent partial resection of the pituitary tumor via a transsphenoidal approach. Pathology was positive for metastatic, poorly differentiated neuroendocrine carcinoma, consistent with small cell carcinoma of the lung. CT of the chest, abdomen, and pelvis revealed a 2.1 × 1.5 cm spiculated lesion in the right upper lobe and a presumed left adrenal gland metastasis.
Postoperative MRI demonstrated a residual enhancing suprasellar component with improved mass effect on the floor of the third ventricle, mammillary bodies, and optic chiasm, as well as near-complete resolution of the edema involving the bilateral optic tracts (Figure 4).
The patient underwent stereotactic radiation therapy to the pituitary mass and chemotherapy with cisplatin and etoposide. A subsequent MRI scan following completion of radiation and 1 cycle of cisplatin and etoposide demonstrated significant reduction in size of the residual enhancing lesion.
DIAGNOSIS
Pituitary metastasis from small cell carcinoma of the lung. Differential diagnosis includes pituitary macroadenoma.
DISCUSSION
Pituitary metastases are rare, most commonly encountered in the 6th and 7th decades of life with no predilection for gender.1 In a recent literature review based on 425 reported cases of primary malignancies metastatic to the pituitary gland, He et al found the incidence of pituitary metastases to be 1.9% among all autopsied cancer cases and 0.9% among all intracranial metastases.2 Breast cancer was found to be the most common primary site at 37.2% followed by lung cancer at 24.2%, prostate cancer at 5.2%, and renal cell cancer at 4.9%.2
The vast majority of pituitary metastases are clinically silent; only 6.8% of cases are symptomatic.3 Of those that are symptomatic, diabetes insipidus (DI) is the most common initial presentation, with one study reporting an incidence of 61.1%.4 Other initial presentations include anterior hypopituitarism, visual disturbances, fatigue, and headache.1,4,5
It has been suggested in many studies that metastasis to the pituitary gland predominantly involves the posterior pituitary, which may explain the high incidence of DI secondary to disruption of vasopressin production.3,4,6 Lau et al demonstrated that posterior pituitary involvement, either alone or in combination with the anterior pituitary, was found in 84.6% of cases, while anterior pituitary involvement alone was seen in only 15.4%.6 The increased incidence of posterior pituitary involvement is thought to be secondary to direct blood flow from the systemic circulation via the portal system;4 the anterior pituitary, on the other hand, derives its blood flow from the pituitary portal vasculature.4 One study, however, demonstrated anterior pituitary involvement to be more common.7
Differentiating a pituitary metastasis from a pituitary adenoma is often difficult, as the clinical and radiologic findings are often nonspecific.8,9 Clinically, one helpful differentiating factor is the initial presenting symptom. As discussed, the most common initial presentation of pituitary metastasis is DI. Pituitary adenomas, on the other hand, present with DI in only 1% of cases.4 Furthermore, the clinical findings most characteristic for pituitary metastasis is rapid onset, invasive growth, diabetes insipidus and/or cranial nerve deficits, and patient older than 50 years.4 In terms of imaging findings, the most characteristic feature of a pituitary metastasis is enlargement or enhancement of the pituitary stalk with a pituitary mass.4 Habu et al found a pituitary stalk mass in 63.7% of patients, a hypothalamic mass lesion in 17.4%, loss of hyperintensity at the neurohypophysis in 82.3%, intratumoral hemorrhage in 8.5%, constriction of the tumor at the diaphragmatic hiatus resulting in a dumbbell shape in 44.7%, hyperintensity around the optic tract in 11.5%, and contrast enhancement of the dura mater around the pituitary fossa in 17.1%.5 Unfortunately, these findings can also be seen in pituitary adenomas and, ultimately, histologic diagnosis is necessary.2,9
Another differentiating feature worth mentioning is T2 signal edema-like change in the optic tracts. Edema-like change has so commonly been associated with craniopharyngiomas that this finding has highly suggested a diagnosis of craniopharyngioma.10,11 Recent studies demonstrate that, although much more commonly seen with craniopharyngiomas, other entities such as pituitary adenomas, germ cell tumors, and malignant lymphomas can also demonstrate similar findings.10,12 Saeki et al described the first case of edema in the optic tracts secondary to pituitary metastases in 2001.12 In their report, they noted a correlation between optic tract edema and the appearance of visual disturbance.12 In a subsequent article, they described the reversibility of this change as evidenced by its disappearance or decrease following successful treatment.10 The authors suggest that this edema-like change is secondary to the distention of Virchow-Robin spaces that occurs when the more distal drainage pathway of Virchow-Robin is blocked.10
Treatment of pituitary metastases includes radiation and chemotherapy.5,13 Surgery, most commonly performed via the transsphenoidal approach, may be considered for symptomatic relief and to improve quality of life; however, it has not been shown to improve survival.4 Regardless, surgery is indicated when the diagnosis is uncertain and when there are symptomatic lesions, particularly those resulting in visual disturbances or pain.4
CONCLUSION
This is a rare case of metastatic small cell lung cancer with initial clinical presentation of bitemporal hemianopsia related to pituitary metastasis. Imaging findings were significant for a sellar/suprasellar mass with edema along the bilateral optic tracts.
REFERENCES
- Siqueria PF, Mathez ALG, Pedretti DB, et al. Pituitary metastasis of lung neuroendocrine carcinoma: case report and literature review. Arch Endocrinol Metab. 2015;59(6):548-553.
- He W, Chen F, Dalm B, et al. Metastatic involvement of the pituitary gland: a systematic review with pooled individual patient data analysis. Pituitary. 2015;18:159-168.
- Teears RJ, Silverman EM. Clinicopathologic review of 88 cases of carcinoma metastatic to the pituitary gland. Cancer. 1975;36:216-220.
- Morita A, Meyer FB, Laws ER. Symptomatic pituitary metastases. J Neurosurg. 1998;89:69-73.
- Habu M, Tokimura, H, Hirano H, et al. Pituitary metastases: current practice in Japan. J Neurosurg. 2015;123:998-1007.
- Lau G, Tan SY, Chiang G, et al. Bronchioloalveolar carcinoma with metastasis to the pituitary gland: a case report. J Clin Pathol. 1998;51:931-934.
- Marin F, Kovacs KT, Scheithauer BW, et al. The pituitary gland in patients with breast carcinoma: a histologic and immunocytochemical study of 125 cases. Mayo Clin Proc. 1992;67:949-956.
- Fassett DR, Couldwell WT. Metastases to the pituitary gland. Neurosurg Focus. 2004;16(4);E8.
- Senetta R, Castellano I, Garbossa D, et al. Pituitary metastasis of an unknown neuroendocrine breast carcinoma mimicking a pituitary adenoma. Pathology. 2013;45(4):422-424.
- Saeki N, Uchino Y, Murai H, et al. MR Imaging Study of Edema-Like Change along the Optic Tract in Patients with Pituitary Region Tumors. AJNR Am J Neuroradiol. 2003;24:336-342.
- Nagahata M, Hosoya T, Kayama T, et al. Edema along the optic tract: useful MR finding for the diagnosis of craniopharyngiomas. AJNR Am J Neuroradiol. 1998;19:1753-1757.
- Saeki N, Murai H, Kubota M, et al. Oedema along the optic tracts due to pituitary metastasis. Br J Neurosurg. 2001;15:523-526.
- Komninos J, Vlassopoulou V, Protopapa D, et al. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004;89(2):574-580.
Citation
DS L, B G, S P, J R, H M.Pituitary metastasis from lung carcinoma presenting as a pituitary adenoma. Appl Radiol. 2018; (7):34-36.
July 6, 2018