Pediatric Appendicitis US: Practical Considerations
CME credits are available here.
Introduction
Appendicitis is a frequent pediatric surgical emergency in the United States, occurring at a rate exceeding 100 per 100,000 person-years.1 It is more frequent in biological men, individuals of White ethnicity, and those ages 10-19.2, 3 Classic symptoms are absent in up to one-third of cases.2 The main causes of inflammation include bacterial overgrowth, lumen obstruction, and ischemic mucosal damage.3 Abdominal pain, especially in the right lower quadrant (RLQ), along with guarding, tenderness, and elevated inflammatory blood markers, including C-reactive protein and white blood cell count, raise clinical suspicion.4 - 6
While many sites perform CT and MRI for suspected appendicitis, US is often the initial imaging modality in staged clinical pathways that incorporate risk stratification owing to its cost-effectiveness, frequently more rapid availability and performance, absence of contrast requirement, and lack of radiation exposure.2, 3, 7 US demonstrated a pooled sensitivity of 72.5% and specificity of 97.0% in one multicenter study.7 However, US is highly operator dependent. Visualization of the appendix may be challenging owing to factors such as operator experience, patient body habitus, excessive bowel gas, and variable appendix location.3, 6 This article describes techniques for the sonographer and radiologist to identify and characterize the pediatric appendix. We focus on how to perform the US study so that the radiologist can do so personally or provide guidance to sonographers who are less experienced in US of the pediatric appendix.
Technique
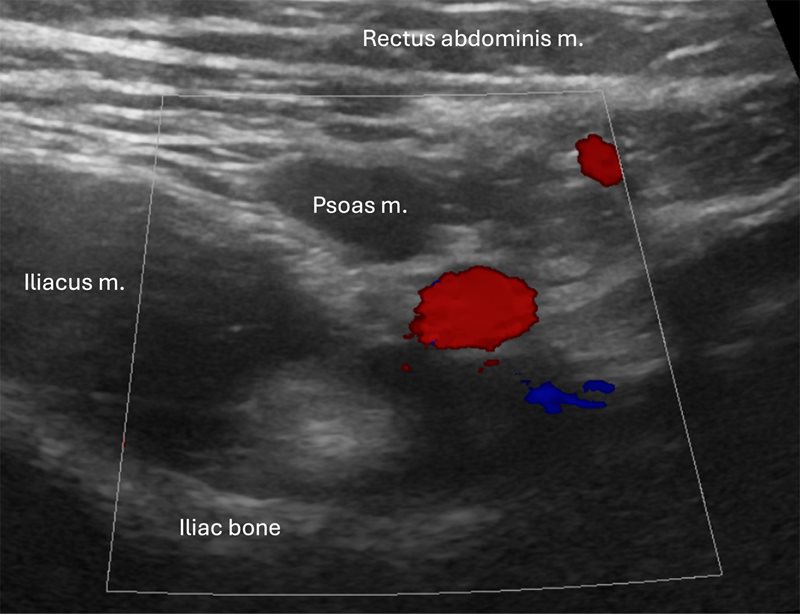
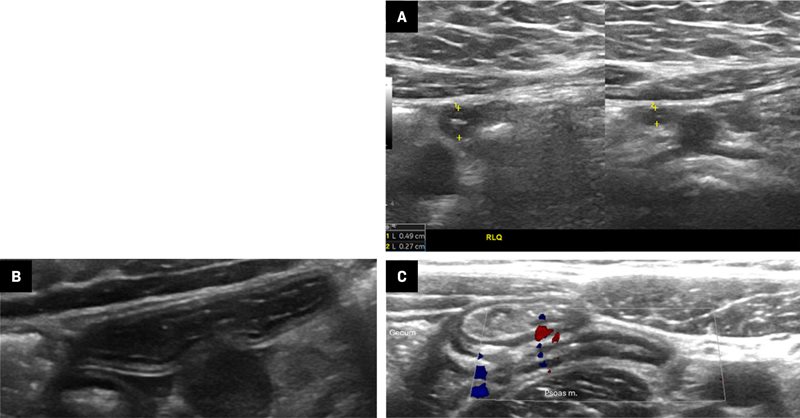
The examination begins by positioning the child supine and asking the patient to indicate the location of maximum pain or tenderness to help identify the inflamed appendix’s position. If a specific point is mentioned, initiate the examination from that spot.2, 3 Distraction techniques, such as using electronic tablets, toys, or pointing to images during the US examination, can be beneficial to help the child remain distracted from any pain and help them keep still. High-frequency transducers (eg, 6-15 MHz linear transducers) are ideal,2, 5 while low-frequency transducers (eg, 1-6 MHz curved array) are suitable for patients with a large body habitus or a deep-seated appendix in whom greater penetration depth is necessary.2, 3 Harmonic imaging should be used if available. An excessively deep field of view decreases axial resolution and the ability to visualize small caliber structures such as the normal appendix (3-5 mm). To facilitate visualization of a normal appendix, initially set the depth of the scan to include the RLQ bowel, but not deeper structures such as the posterior aspect of the iliac wing and, in younger children, the vertebrae. Start scanning with light to moderate pressure, applying additional pressure on expiration.3 Normal bowel can be displaced by a technique called graded compression. This technique involves applying light pressure with the transducer during the initial anterior abdominal sweep.8 The goal is to displace and compress bowel gas and fluid from the cecum and the terminal ileum (TI) with improved visualization of the noncompressible inflamed appendix with gradually increasing pressure.8 Important anatomic landmarks include the cecum, TI, psoas muscle, and iliac vessels ( Figure 1 ). The appendix is typically found inferior to the cecum and TI, and anterior to the psoas muscle and iliac vessels.
The iliac vessels (red and blue) and psoas major muscle are helpful landmarks. This transverse image shows the posterior iliac bone. The depth of field should be reduced to better visualize small structures such as the appendix.
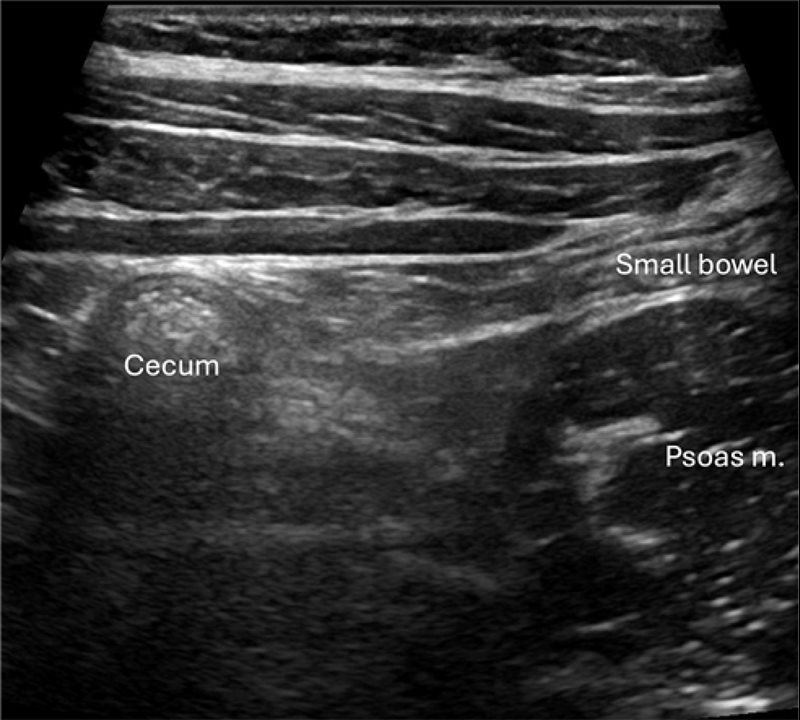
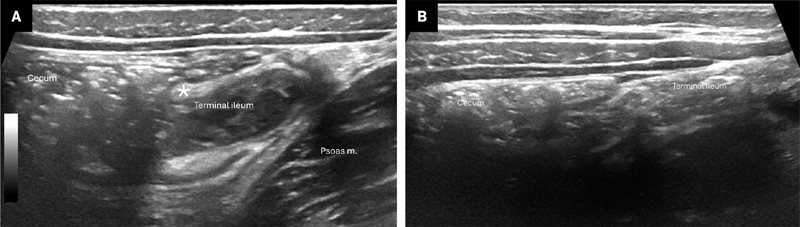
Our practice is to first identify the ascending colon and cecum in the transverse plane ( Figure 2 ). Compared with the small bowel, the colon is located more laterally, contains more air, often resulting in “dirty” shadowing,9 and is generally of a larger caliber, making it relatively easy to identify in many patients. Second, we move the transducer inferiorly to attempt to locate the TI and ileocecal valve (ICV) ( Figure 3 ). The appendix usually arises from the cecum on the same side as the ICV and 2-3 cm inferior to the ICV, making visualization of the ICV a helpful landmark.2, 3 Identifying the TI is additionally useful to exclude potentially confounding diagnoses (eg, inflammatory bowel disease) and ensure that the TI is not mistaken for the appendix. The TI should be documented with grayscale and color Doppler imaging to evaluate for hyperemia. The appendix is variably positioned and, in more than 50% of cases, it is retrocecal.10 In addition to graded compression to help identify the appendix, posterior manual compression aids in reducing the distance from the transducer to the bowel, facilitating visualization, especially in patients with larger abdomens.2, 3 Placing the patient in a left lateral decubitus can help position the cecum and TI medially, improving access to the retrocecal region.3 If the appendix remains occult, repositioning the patient may cause bowel gas movement, enhancing visualization.2 Some have suggested adding a posterior approach.11
Grayscale image of the right lower quadrant shows the cecum in the transverse plane. The colon (seen here in cross-section) is located laterally, contains air with “dirty” shadowing, and is larger caliber than the adjacent small bowel (shown longitudinally).
When possible, radiologists and sonographers should locate the terminal ileum (TI) and cecum. The ileocecal valve (ICV) (*) is variably identified and visible in (A) but not (B).
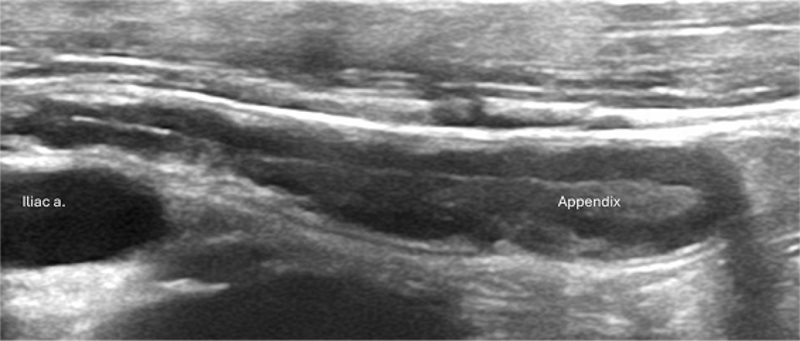
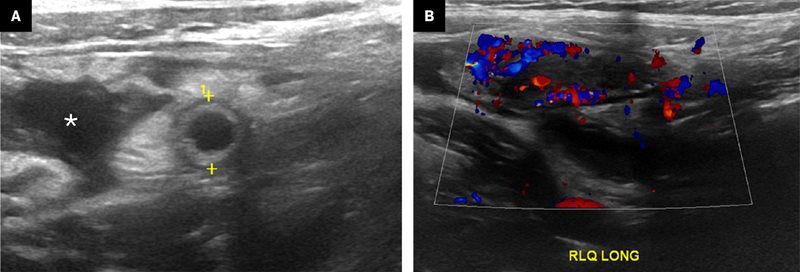
Once identified, the appendix should be documented in grayscale and color Doppler images. The Doppler pulse repetition frequency should be very low. Longitudinal images showing the blind-ending tip ( Figure 4 ) and, if possible, origin from the cecum will prove that the structure identified is the appendix and not another piece of bowel ( Figure 5 ). The presence of hyperemia is best demonstrated in longitudinal images. Compression images showing transverse luminal diameter should be obtained side-by-side ( Figure 5 ).
Longitudinal image of a normal appendix (calipers) with expected wall structure and echogenic luminal contents
Transverse images ( A ). Compressibility should be assessed by measuring the diameter of the appendix, ideally in a side-by-side grayscale view (left, without compression; right, with compression). Longitudinal images showing the cecum origin in grayscale ( B ) and the blind-ending tip on color Doppler ( C ). Note that the appendix is not displaced from the adjacent structures by inflamed fat or edema; there is no fat stranding.
Morrison pouch and the pelvis are commonly examined with low-frequency transducers and a deeper field of view to evaluate for free fluid or abscess.3 The appendix may be seen suspended within any free fluid.2
Normal Appendix
The normal appendix is a compressible, blind-ending, tubular structure featuring 5 distinct layers in the wall, although only 3 may be visible. The innermost layer is a hyperechoic mucosal linear structure containing lymphoid tissue.2 Appendiceal diameter is typically less than 6 mm and does not change with age.12 The maximal mural thickness, however, does vary with age and a maximum of 3 mm should be considered normal for those under 6 years old.12, 13 There should be minimal color Doppler signal in the appendix wall ( Figure 5 ). Gas in the appendix typically indicates the absence of acute appendicitis.14 The appendix is often enlarged in patients with cystic fibrosis in the absence of appendicitis (mean diameter of 8.3 mm).15 Visualization of the normal appendix in its entire length, including its tip, definitively excludes appendicitis.16 However, an appendiceal US in which the appendix is not visualized and no inflammatory findings are present in the RLQ has been shown to have a high negative predictive value.7
Acute Appendicitis
Appendix thickening, maximal tenderness over the thickened appendix, noncompressibility, (large) appendicolith, and hyperemia ( Figure 6 ) are the primary US indicators of appendicitis.16 While 6 mm is the conventional cut-off for appendiceal diameter for US, it has been suggested that specificity may be improved with a cut-off of 7 mm.17 Note that this cut-off value does not apply to CT or MRI.
Primary signs of appendicitis ( A ). Calipers are placed on the outer walls of the dilated, fluid-filled, hyperemic appendix. Note the displacement of the appendix away from adjacent structures by the thickened, echogenic surrounding fat stranding, a secondary sign of appendicitis. Free fluid (*) is also present. Color Doppler ( B ) shows hyperemia within the appendiceal wall, a primary sign of appendicitis. Free fluid is also demonstrated.
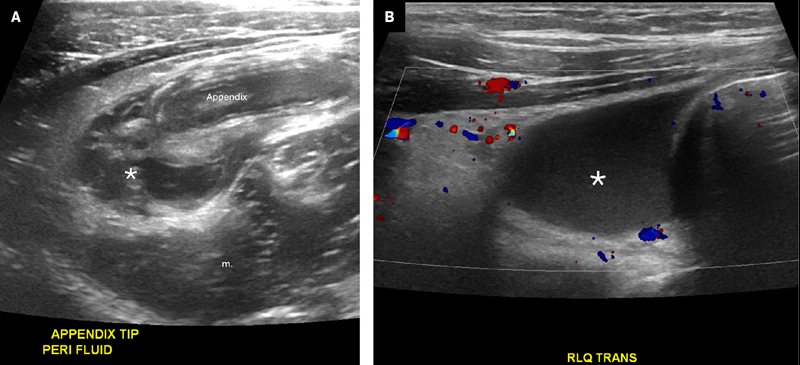
Secondary signs of appendicitis include RLQ mesenteric fat stranding, the presence of a complex fluid collection, mesenteric lymphadenopathy, and/or periappendiceal fluid.16 Fat stranding appears as mesenteric thickening and hyperechogenicity ( Figure 7 ). Hyperechoic, thickened fat is highly specific for inflammatory disease in the RLQ.18, 19 An appendicolith has been variably associated with acute appendicitis.20 An additional concerning finding is the presence of an excessive volume of free abdominopelvic fluid. A small amount of simple free fluid has low specificity for appendicitis in both boys and girls, but a moderate to large amount has been reported to be highly specific.19 Complex fluid is highly specific for perforated appendicitis.20 Focal, complex fluid suggests the presence of an abscess ( Figure 7 ).
Grayscale US image ( A ) of the right lower quadrant (RLQ) shows a dilated, inflamed appendix with surrounding fat stranding and an adjacent complex fluid collection (*) in a patient with a perforated appendicitis and abscess. Color Doppler image ( B ) of the RLQ shows complex free fluid (*), hyperemia, and fat stranding.
Differential Diagnosis and Pitfalls
Various conditions can mimic appendicitis, including enterocolitis, inflammatory bowel disease, appendiceal lymphoid hypertrophy, mesenteric adenitis, Meckel diverticulum, and tumor. Enterocolitis and inflammatory bowel disease are causes of RLQ pain that can mimic appendicitis. It is crucial to recognize the cecum and ilium and differentiate them from the appendix. The TI, ending at the ICV, is not blind-ending and exhibits peristalsis. There may be reactive inflammation of the cecum and ileum in acute appendicitis, but the amount of inflammation tends to be relatively minor. Conversely, inflammatory bowel disease is often most severe at the TI. Involvement of the appendix can be seen relatively frequently in Crohn disease.16
Lymphoid hyperplasia is identified by clusters of more than 10 lymphoid nodules with follicles exceeding 2 mm in size. This condition can reduce the compliance of the appendiceal wall, leading to noncompressibility on US and increasing the appendix’s maximum diameter beyond 6 mm, which may result in a false-positive diagnosis of appendicitis.21 Distinguishing between lymphoid hyperplasia and appendicitis is aided by identifying periappendiceal fluid collection and a lamina propria thickness of 1 mm or less, which are considered the most reliable indicators.21
Mesenteric adenitis can be identified by reactive mesenteric lymph nodes, with a short axis of more than 5 mm as well as more than 3 lymph nodes in the small bowel mesentery without acute inflammation.3, 22, 23 Meckel diverticulum is a blind-ending structure arising from the distal ileum, often associated with rectal bleeding. Meckel diverticulitis should be suspected if a cyst-like structure with gut US signature is identified and the structure has anomalous vessels and signs of wall inflammation on color Doppler.24 Origin of the inflamed structure from the cecum excludes a diagnosis of Meckel diverticulum.25
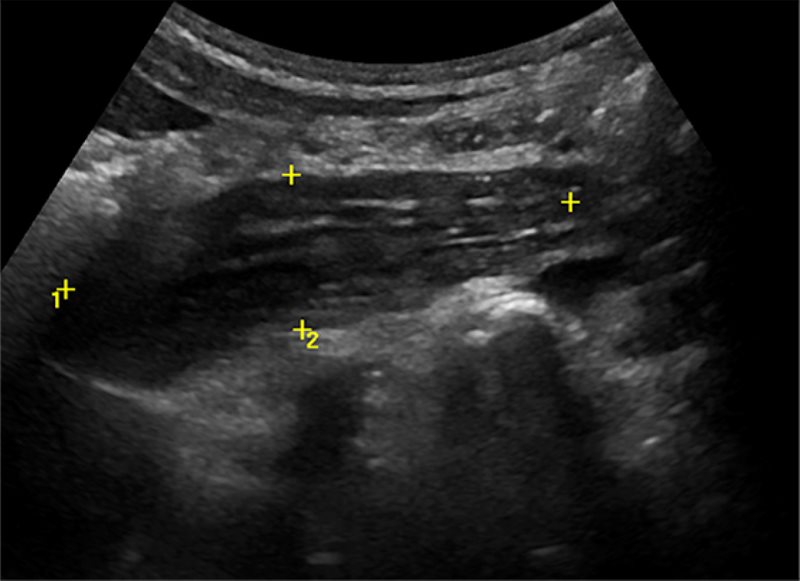
Other tubular-appearing structures in the RLQ can mimic an inflamed appendix. The psoas muscle, when imaged obliquely, can be mistaken for an inflamed appendix ( Figure 8 ).16 Tuboovarian abscess could be mistaken for a perforated appendicitis.26
Grayscale US image shows calipers placed around the iliopsoas muscle, which can be mistaken for an inflamed appendix
Reporting Right Lower Quadrant US
After obtaining the patient’s history and employing the appropriate technique, the report should assess the appendix, detailing its diameter, compressibility, Doppler flow, presence of appendicolith, and any secondary signs. Additionally, the report should describe findings such as other bowel wall thickening or enlarged lymph nodes. Structured reporting may decrease the use of CT and reduce the rate of negative appendectomies.27 If the appendix is not identified and any secondary signs are present, CT or MRI should be performed. In cases where clinical suspicion remains high despite a negative or inconclusive US, referrers may opt to perform further imaging studies, such as CT or MRI, before arriving at a definitive diagnosis or ruling out appendicitis.16, 28
Conclusion
Appendicitis is the most prevalent surgical emergency in children. The techniques described in this article should aid the radiologist and sonographer in performing an US examination for appendicitis and the radiologist in interpreting the findings.
References
Citation
Hayrapetian A, Jeyasingam M, Francavilla M.Pediatric Appendicitis US: Practical Considerations. Appl Radiol. 2024; (6):6 - 11.
doi:10.37549/AR-D-24-0039
December 1, 2024