Partnership Expands Lung Screening Capabilities
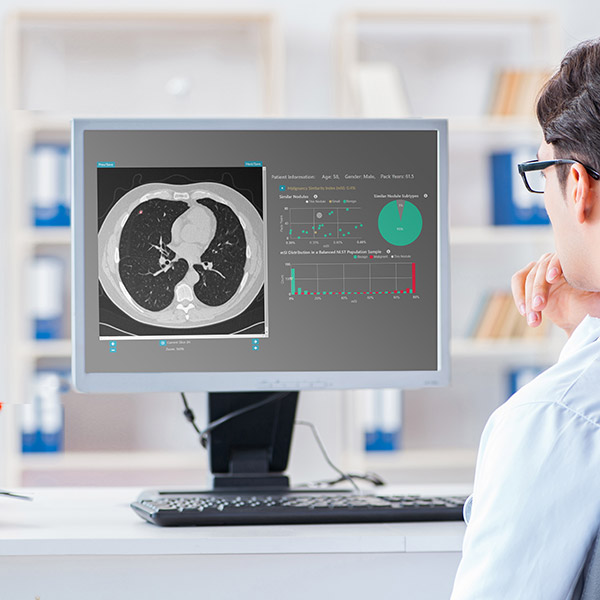 Volpara Health and Seattle-based lung AI company RevealDx are partnering to further expand Volpara’s lung screening capabilities and potentially detect more lung cancers earlier.
Volpara Health and Seattle-based lung AI company RevealDx are partnering to further expand Volpara’s lung screening capabilities and potentially detect more lung cancers earlier.
Approximately 20%-30% of lung CT exams reveal lung nodules that need to be characterized as benign or malignant. The workup typically may include a follow-up lung CT exam in a few months or a surgical biopsy. In addition, many lung nodules are found incidentally when CT exams are performed for other reasons, such as shortness of breath, chest pain or injury.
Volpara Lung software – an advanced, integrated, and adaptable reporting, tracking, and risk assessment solution for lung cancer screening – enables patient management from scheduling to diagnosis. The software offers structured reporting, customizable reminders, and more than 40 statistical reports to monitor and track patients – critical for outreach to patients overdue for their screening or follow-up exams. Volpara Lung automatically collects and validates all the required data elements for successful submission to the American College of Radiology Lung Cancer Screening Registry for reimbursement.
RevealDx, formerly known as Mindshare Medical, is focused on improving lung cancer outcomes by delivering the most advanced, radiomics and AI-enabled, lung cancer decision-support software to drive the optimal clinical pathway for each patient based on automated analytics of each lung nodule. Its patented, CE-marked software product integrates with picture archiving and communication systems (PACS) and scores nodules found in lung CT, informing better clinical decision making. Early clinical data for RevealAI-Lung suggests that many cancers can be detected earlier by using the AI nodule analysis rather than waiting for a scan in a few months’ time, while simultaneously reducing false positives, with potentially significant cost savings.
“When we acquired MRS, we entered the lung cancer screening market with practice management software covering about 8% of the US lung cancer screening market. Since then, the benefits of lung cancer screening using low-dose CT have become more widely accepted globally, with the US doubling the number of people eligible, and countries like Australia starting the process of scoping out nationwide programs. Despite the clear benefits of screening in reducing mortality, a major issue those programs face is the number of false positive nodules found in routine screening as well as incidental findings. That differentiation of nodules is exactly what RevealDx has been focusing on. We look forward to working closely with them to bring that technology to market as part of an integrated lung cancer screening platform,” said Ralph Highnam, Ph.D., Group CEO of Volpara.
“Volpara has a solid market share in lung, and we look forward to working closely with them to fully integrate RevealDx’s AI capabilities into a wider lung offering both here in the United States and into Australia and New Zealand. By informing precision medicine, our products will improve follow-up for millions of patients. The ability to diagnose early, inform treatments, and optimise the total cost of care will be a game-changer,” said Chris Wood, RevealDx CEO.