MRI-Based Deep Learning Predicts Biomarkers of Alzheimer Disease
Images
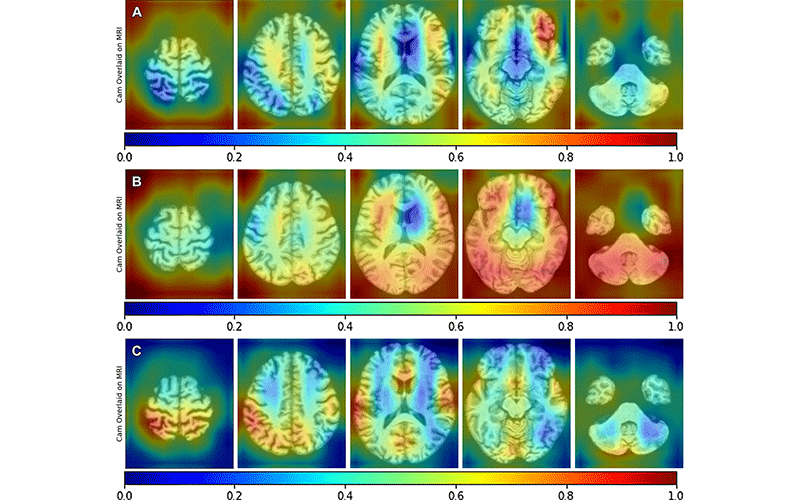
According to new research in Radiology, applying the power of deep learning to brain MRI can help predict neurodegeneration and other markers of Alzheimer disease (AD) years before symptoms start,
Once identifiable only through post-mortem examination, AD today is linked with several biomarkers in living patients, including:
- amyloid, a naturally occurring protein that clumps together in AD to form plaques that impair brain function.
- tau, another naturally occurring protein that in AD sticks together to form tangles that block communication between neurons.
- neurodegeneration, a loss of nerve cells in the brain.
The presence or absence of these biomarkers is used in the Amyloid-Tau-Neurodegeneration (ATN) classification system. ATN classification has taken on increasing importance now that AD is understood as a heterogeneous disease in which patients with similar clinical presentations could have very different underlying pathophysiologies.
“Depending on what subgroup theyʼre in, some patients may respond better to therapy than others,” said Jeffrey R Petrella, MD, director, Alzheimer Disease Imaging Research Laboratory and professor of radiology at Duke University Medical Center, Durham, NC. “Moreover, the type of therapy patients respond to may also differ across subgroups.”
Several imaging options have become available over the past decade to diagnose AD and to determine the stage of the disease. For example, PET can be used for ATN classification, but it incurs considerable cost and exposure to ionizing radiation for patients. Using spinal taps to assess cerebrospinal fluid biomarkers incurs some risk and may not be acceptable for some patients and their referring physicians.
MRI presents a noninvasive option for biomarker detection, but it currently has limited use in characterizing ATN status. It can show the neurodegeneration that occurs later in AD but cannot detect amyloid accumulations that is an earlier sign.
“Thatʼs where deep learning comes in,” said Dr Petrella, who has been researching AD for more than 20 years and gave the RSNA 2011 Annual Oration in Diagnostic Radiology entitled, Neuroimaging and the search for a cure for Alzheimer disease. “Deep learning can extract patterns from the data that may not be apparent with the naked eye. We can train a model to extract these hidden features and use them in the most effective way possible to get information about biomarkers.
Dr Petrella and colleagues applied deep learning algorithms to MRI images to predict PET determined ATN biomarker status.
The MRI and PET data came from the Alzheimerʼs Disease Neuroimaging Initiative (ADNI), a public-private partnership focused on developing biomarkers for the early detection and tracking of AD. The data set included 2,099 amyloid, 557 tau, and 2,768 fluorodeoxyglucose (FDG) PET-MRI scan pairs. The patients had diagnoses across the cognitive spectrum at the time of imaging. Some patients were cognitively normal, others had AD and others had mild cognitive impairment, a condition characterized by memory and thinking problems that is linked with a higher risk of AD.
Using MRI and other readily available diagnostic data, the deep learning algorithm predicted each component of PET-determined ATN status in AD with promising results, the efficacy depending on the biomarker (area under ROC for amyloid 0.79; tau 0.73; neurodegeneration 0.86). There was a significant improvement in performance of the combined MRI plus diagnostic data model compared to diagnostic data alone (DeLong P < 0.005).
“Most patients undergoing a dementia workup already have an MRI scan along with a Mini Mental Status Exam score and basic demographics on their medical chart,” Dr Petrella said. “The idea is to take this readily available data and try to extract as much information as we can to predict these important biomarkers.”
The researchers emphasize the need for further studies to validate their findings in larger and more diverse community-based patient populations. However, these biomarker predictions have the potential to transform AD prognosis and treatment planning.
For example, patients who are amyloid positive and with mild cognitive impairment will decline at a faster rate than those who are amyloid-negative, making early predictions of amyloid status crucial for optimal treatment. The MRI-deep learning approach also may have applications for determining eligibility for the new class of FDA-approved anti-amyloid therapies now available.
Additionally, with these encouraging results, the researchers are seeking funding for the next planned project that includes the addition of plasma biomarkers to improve the accuracy rates of MRI and deep learning to over 95%.
Dr Petrella said the study provides another example of the value of ADNI, an initiative that has expanded internationally since its 2004 launch in the US.
“ADNI has really been a big service to us and other scientists,” Dr Petrella said. “Itʼs enabled us to use our methodology in patients across many different centers and MRI scanner types and field strengths. This allows us to generalize our conclusions more broadly than we otherwise could if the study were conducted at a single center.”
Related Articles
Citation
MRI-Based Deep Learning Predicts Biomarkers of Alzheimer Disease. Appl Radiol.
May 28, 2024