Lumbar Spine AI Software for MRI Receives FDA Clearance
 SmartSoft Healthcare has received US FDA clearance for CoLumbo, a lumbar spine AI software for MRI reading and reporting.
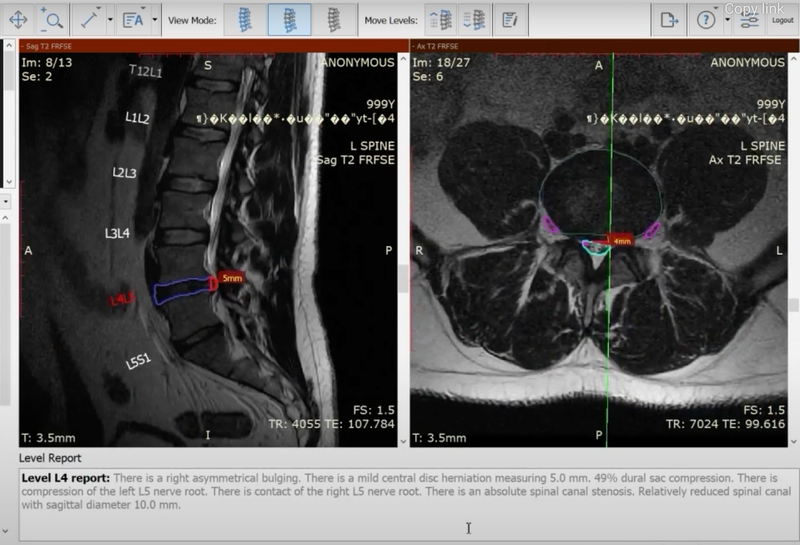
SmartSoft Healthcare has received US FDA clearance for CoLumbo, a lumbar spine AI software for MRI reading and reporting.
CoLumbo is deep learning software, based on a Fully Convolutional Neural Networks (FFCN) architecture. It is an assistant type of software that identifies and measures all major structures of the lumbar spine, through automatic segmentation, and provides a detailed description of findings in an editable draft report. The solution also classifies MRI images to provide pathology description, findings and measurements with pre-populated structured or free text report.
According to the company, CoLumbo reduces the time needed to read images and report findings by up to 25% with no loss in accuracy and reduces errors of omission by up to 15%. CoLumbo has successfully passed clinical trials that were held in three European University hospitals with 382 patients. It has also obtained a CE mark certification, making CoLumbo approved for clinical use in Europe and the US.
“CoLumbo is a powerful tool that impacts the quality and value of lumbar spine MR reporting, assisting in standardization, completeness and consistency,” said Lawrence Tanenbaum, MD, FACR Chief Technology Officer, Vice President, RadNet Inc.
Related Articles
Citation
Lumbar Spine AI Software for MRI Receives FDA Clearance. Appl Radiol.
June 29, 2022