Infections and inflammatory conditions of the pediatric spine and spinal cord
Images






Infectious and inflammatory conditions of the pediatric spine are uncommon and often nonspecific. However, in certain instances,such as epidural abscess, quick diagnosis and treatment are imperative. The aim of this paper is to briefly review pediatric inflammatory and infectious lesions of the spine and spinal cord with emphasis on the features that render specific diagnosis.
Idiopathic transverse myelitis
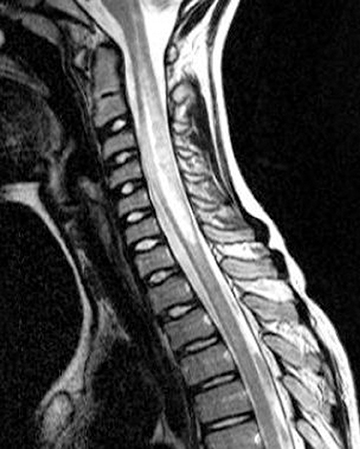
Patients with transverse myelitis (TM) present with paresthesias, pain, bilateral leg weakness, sphincter dysfunction, and a clearly defined sensory level, which typically progresses over the course of 4 hours to 21 days. If the symptoms progress rapidly (within4 hours) this is more suggestive of ischemic myelopathy. The diagnosis of idiopathic acute transverse myelitisis supported by cerebrospinal fluid (CSF) pleocytosis (usually elevated white blood cells) and elevated IgG index. Idiopathic transverse myelitis is a diagnosis of exclusion, as other etiologies can cause transverse myelitis. These include prior radiation (within the last 10 years),anterior spinal artery thrombosis, spinal arteriovenous malformation, connective tissue disease, or CNS infection.
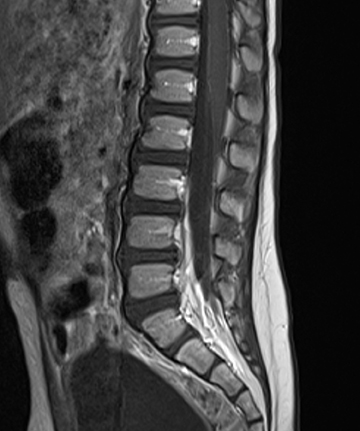
Idiopathic acute transverse myelitis is seen on MRI as a T2W hyperintensity involving most of the axial cord spanning more than one vertebral body segment, with or without cord expansion and/or enhancement (Figure 1). The T2W hyperintensities tend to be more central and uniform compared to the patchy peripheral hyperintensities seen in multiple sclerosis (MS). In the recovery phase, this long spinal cord hyperintensity may split up into several smaller distinct lesions.1
Neurologists may use the 2002 Transverse Myelitis Consortium Working Group criteria for transverse myelitis, which defines gadolinium enhancement as one of the criteria for diagnosing transverse myelitis, even though this is not supported within the radiology literature.2 These guidelines also suggest repeat MRI scans within a week if the first MRI is nondiagnostic and there is persistent clinical concern. A brain MRI should also be performed to see if the transverse myelitis really is idiopathic, or if other white matter lesions such as multiple sclerosis (MS), acute disseminated encephalomyelitis (ADEM) or optic nerve involvement (neuromyelitis optica) could explain the spine findings.
Neuromyelitis optica
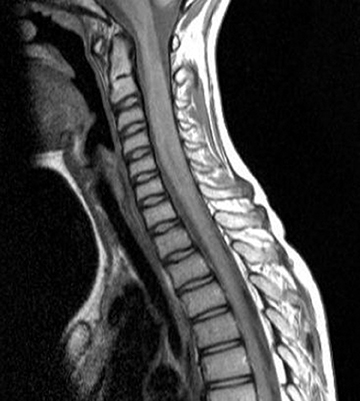
Neuromyelitis optica (NMO), or Devic’s disease, is seen in patients presenting with optic neuritis and transverse myelitis (concurrently or subsequently). In children, NMO is more commonly seen in nonwhite children and children with systemic autoimmune diseases, such as systemic lupus erythematosus.3 The two most common symptoms of optic neuritis are vision loss and eye pain, and children are more likely than adults to present with bilateral optic neuritis.4,5 The most specific indicator for NMO is a serum marker, neuromyelitis optica immunoglobulin G (NMO-IgG), which is directed against aquaporin-4 (AQP4-antibody), and can aid in early diagnosis.6 The typical CSF findings are elevated proteins and neutrophils. Studies on AQP4-antibody positive patients have clarified that brain lesions are not uncommon in pediatric NMO patients.3
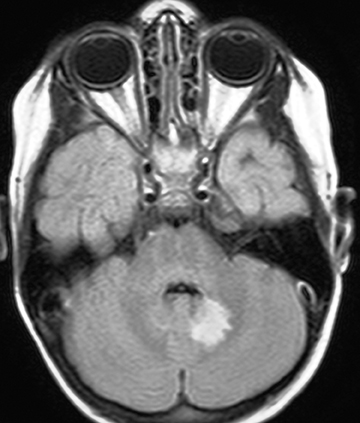
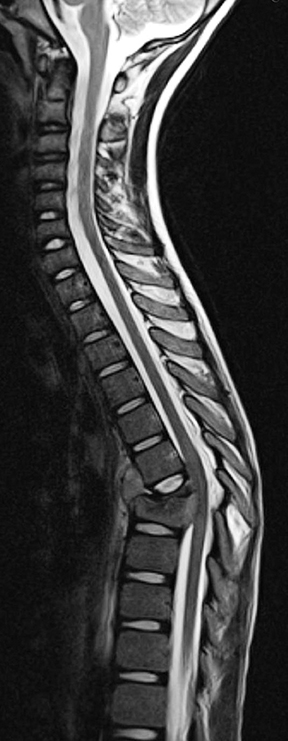
On MR imaging, in contrast to MS, which demonstrates smaller, peripherally located spinal cord lesions, NMO shows intramedullary T2W hyperintensity (usually > 3 vertebral bodies in length) typically located centrally and with or without contrast enhancement (Figure 2). The contrast enhancement can mimic an intramedullary tumor, but unlike a tumor, NMO tends not to cause cord expansion. Some of the brain MRI lesions seen in NMO appear to be specific, such as those seen in the hypothalamus and periaqueductal region surrounding the ventricular system.7 Neurologists may use the 2007 consensus criteria for CNS demyelination in children, which requires both optic neuritis and transverse myelitis and either a spinal lesion extending over 3 or more segments or NMO-IgG positive serum to make the diagnosis.8
Acute disseminated encephalomyelitis (ADEM)
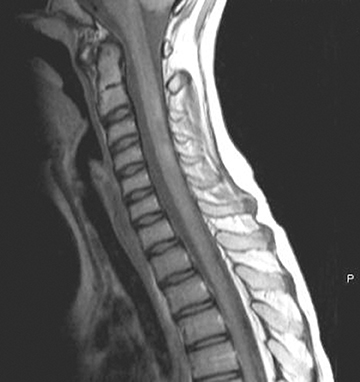
Acute disseminated encephalomyelitis (ADEM) is an encephalopathy seen a few days to several weeks after a viral illness or vaccination.8 Patients present with acute onset of headache, seizures, nuchal rigidity, acute hemiparesis, cerebellar ataxia, cranial nerve palsy, visual loss, and altered mental status.1 The brain is the most commonly involved in ADEM patients with spinal cord disease in about a quarter of patients.1 Children affected with ADEM are usually younger than 10-years-old.9 ADEM can be monophasic, recurrent (second attack involving the same areas >3 months from the first attack), or multiphasic (second event in a different area).8 Up to 25% of patients diagnosed with ADEM are subsequently diagnosed with MS.10
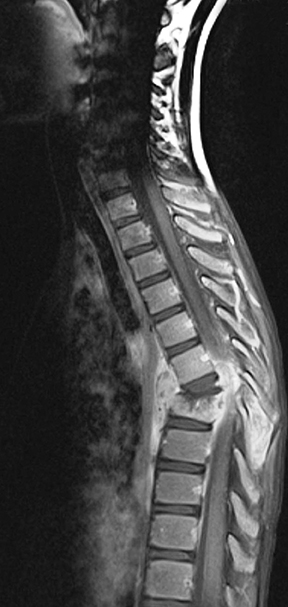
Spinal cord ADEM usually presents with poorly marginated T2W hyperintensities of 2 or 3 vertebral bodies in length (Figure 3). It is unusual for ADEM to show contrast enhancement. The CSF often shows increased protein or leukocytosis. The patient’s clinical symptoms often improve before the resolution of MR imaging findings. A few diseases can show similar spinal cord presentations, including collagen vascular disease, Whipple disease, viral encephalitis, neurosarcoidosis, and MS.1
Multiple sclerosis
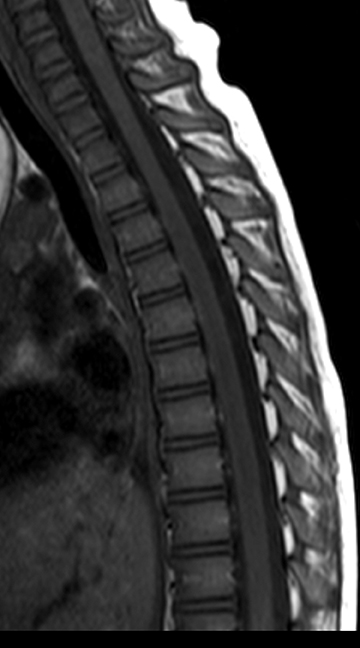
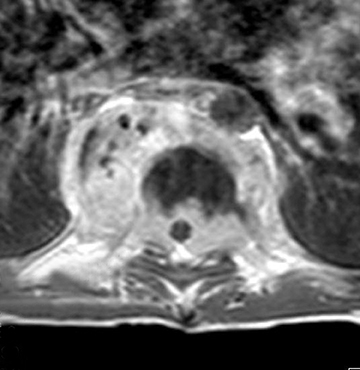
MS is thought to be an adult disease, but approximately 10% of adults with MS have onset of symptoms in childhood.11 The revised McDonald criteria (5 or more T2W hyperintensities, 2 or more periventricular lesions, and 1 brainstem lesion) have a high sensitivity and specificity for children ≥11 years without ADEM features.10,12 One of the most specific findings is oligoclonal bands in the CSF, which is seen in > 90% of cases.13 MS plaques in the spinal cord are typically smaller than TM lesions, patchy, poorly marginated and usually involving the posterolateral cord. Acute lesions can show mass effect and enhancement (Figure 4).
Tumefactive spinal cord plaques may be associated with swelling and mimic neoplasm. Serial imaging and an incomplete rim of enhancement may help to discriminate tumor from tumefactive MS.14 Other diseases and conditions can mimic MS plaques in the spinal cord;they include small infarctions (anti-phospiolipid syndrome, systemic lupus, Takayasu disease, arterial dissection), ADEM, TM, and infections/inflammatory disorders, such as Lyme disease or neurosarcoidosis.1 Meningitis
The classic history for meningitis is altered mental status, fever, and stiff neck; however, any febrile infant is presumed to have meningitis until the CSF has been evaluated. Children can acquire meningitis from hematogenous spread, direct trauma, or local extension. The diagnosis is typically made by lumbar puncture alone (elevated neutrophils/positive cultures). Inflammatory cells infiltrate the arachnoid layer and coat the surface of the spinal cord and the nerve roots, which enhance with contrast on MRI. This appearance can mimic leptomeningeal carcinomatosis.
Guillain-Barre syndrome
Guillain-Barre syndrome (GBS) is seen more commonly in boys than girls, and typically between the ages of 4 and 12. Classically these patients have a viral prodrome of GI or respiratory disease 2 weeks prior to onset of GBS symptoms. These patients have ascending paralysis, which starts with lower extremity weakness and can eventually involve the diaphragm, upper limbs, and cranial nerves. Analysis of CSF will show increased protein in the acute phase. If weakness lasts for more than 2 months, the term chronic inflammatory demyelinating polyneuropathy (CIDP) is used. Guillain-Barre typically lasts 2 to 18 months.
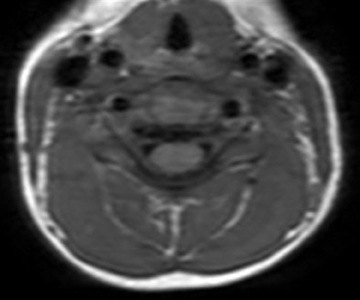
Spinal MRI scans will show enhancement of thickened nerve roots (anterior > posterior) and the cauda equina (Figure 5).15 Early in the course of disease, enhancement can be very mild or even nonappreciable.
Chronic inflammatory demyelinating polyneuropathy
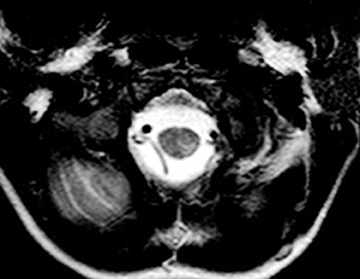
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an acquired, immune-mediated peripheral neuropathy that targets the myelin sheaths of peripheral nerves.16 CIPD has a very low prevalence—48 per 100,000—and a better prognosis in childhood than in adults.17,18 It is characterized by chronic progressive or relapsing and remitting weakness, and sensory loss in multiple limbs.16 Typical findings on spinalMRI are thickening and contrast enhancement of the sacral nerve roots and the cauda equina (Figure 6). The rapid progression of symptoms and the relapsing course, as well as the enlargement of the lumbosacral nerve roots, differentiates CIPD from other diagnoses, such as Guillain-Barre syndrome and hereditary motor and sensory neuropathy (HMSN).Spondylodiscitis
Children (usually 1-5 years old) with spondylodiscitis present with back pain, a limp, irritability, fever, and refusal to walk.19 The white blood cell count and the blood cultures may be negative in these children, although the sedimentation rate is often elevated.19 Children are thought to be more susceptible than adults to spondylodiscitis due to increased lympatics/blood vessels within the disc and endplate. Pediatric spine infection is now thought to start in the vertebral bodies and extend towards the discs.20 The most common bacteria is Stapholococcus aureus and Streptococcus, although no organism is identified in 70% of cases because these patients are treated empirically.21
Magnetic resonance (MR) imaging findings suggestive of spondylodiscitis include narrowed disc space, endplate blurring, and T2W hyperintensity and/or enhancement of the disc or the vertebral body, with or without associated abscess (Figure 7). The best sequence to look for blurring of the endplates is the T1W sequence. If the disc and vertebral body are T2W hyperintense, a disc space abscess with adjacent osteomyelitis should be suspected, especially if the vertebral body enhances with contrast.22 After treatment, abnormal signal can persist in the vertebral body and disc up to 24 to 34 months later, respectively.23 The best predictor of response is lack of disease progression. If there is extensive erosive destruction of the vertebral body with an associated soft-tissue mass consider tuberculosis or coccidioidomycosis spondylitis.
Spinal tuberculosis
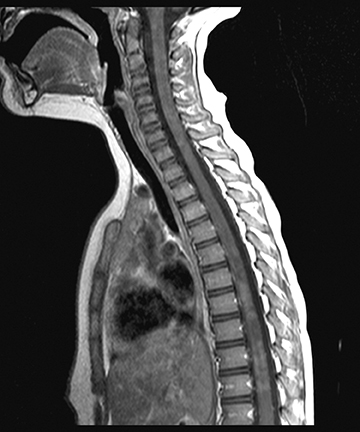
Spinal tuberculosis (TB) is a hematogenously disseminated infection of one or more vertebral bodies with Mycobacterium tuberculosis, with or without spinal cord involvement. Typical presenting features of spinal tuberculosis are back pain and/or symptoms of systematic disease,such as fever, night sweats, anorexia, and weight loss. The thoracic and lumbar spine are most commonly affected. Typical spinal MRI findings are sparing of the intervertebral discs with T2W hyperintensity and T1W hypointensity along the anterior vertebral body (usually with an associated paravertebral component) and postcontrast enhancement (including subligamentous spread).24 The two most serious complications of spinal TB are severe kyphosis and cord compression from epidural extension (Figure 8).
Spinal epidural abscess
Spinal abscesses in children are rare but important to recognize because they require rapid treatment to avoid neurologic injury. The children who are the most susceptible to spinal abscesses are those with indwelling catheters, sepsis, or spinal hardware. A typical clinical presentation of spinal abscess is tender back pain worsened by straining, which develops into radicular pain and spinal cord compression(weakness and loss of sphincter control).1
Spinal empyema is typically seen along the dorsal canal unless associated with a vertebral body. On MRI, a spinal epidural abscess shows enhancement of the solid component with a peripheral rim of enhancement surrounding the liquefied pus.25 Dural enhancement and epidural venous plexus/basivertebral venous engorgement can also be seen (Figure 7).25 Changes in the size of the abscess typically correlates with patients status; however, persistent enhancement is common regardless of the patient’s condition.
Conclusion
MR imaging is the most efficacious diagnostic method to differentiate pediatric inflammatory lesions from infectious lesions of the spine and spinal cord in the pediatric population, revealing particular features that, when combined with clinical and laboratory findings, allow specific diagnoses.
REFERENCES
- Barkovich AJ, Raybaud C. Pediatric neuroimaging. 5th ed. New York, NY: Raven Press; 2011.
- Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002; 59:499-505.
- McKeon A., Lennon VA, Lotz T et al. CNS aquaporin-4 autoimmunity in children. Neurology. 2008;71:93-100.
- The clinical profile of optic neuritis. Experience of the optic neuritis treatment trial. Optic neuritis study group. Arch Ophthalmol. 1991; 109:1673-1678.
- Wilejto M,Shroff M, Buncic JR, et al. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006; 67:258-262.
- Lotze TE, Northrop JL, Hutton GJ, et al. Spectrum of pediatric neuromyelitis optica. Pediatrics. 2008;122: e1039-1047.
- Jacob A, McKeon A, Nakashima I, et al. Current concept of neuromyelitis optica (NMO) and NMO spectrum disorders. J Neurol Neurosurg Psychiatry. 2012 [Epub ahead of print].
- Krupp LB, Banwell B, Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16 Suppl 2):S7-12.
- Banwell B Ghezzi A, Bar-Or A, et al. Multiple sclerosis in children: Clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007;6:887-902.
- Callen DJ, Shroff MM, Branson HM et al. MRI in the diagnosis of pediatric multiple sclerosis. Neurology. 2009;72:961-967.
- Renoux C Vukusic LH, Mikailoff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007; 356:2603-2613.
- Sadaka Y, Verhey LH, Shroff MM, et al. 2010 McDonald criteria for diagnosing pediatric multiple sclerosis. Ann Neurol. 2012;72:211-223.
- Tortori-Donati P, Rossi A. Pediatric Neuroradiology. New York: Springer; 2005:1716.
- Glasier CM, Robbins MB, Davis PC, et al. Clinical, neurodiagnostic, and MR findings in children with spinal and brain stem multiple sclerosis. AJNR Am J Neuroradiol. 1995;16:87-95.
- Yikilmaz A, Doganay S, Gumus H, et al. Magnetic resonance imaging of childhood Guillain-Barre syndrome. Childs Nerv Syst. 2010;26:1103-1108.
- Pollard JD. Chronic inflammatory demyelinating polyradiculoneuropathy. Curr Opin Neurol. 2002;15:279-283.
- McLeod JG, Pollard JD, Macaskill P, et al. Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Ann Neurol.1999;46:910-913.
- Ryan MM Grattan-Smith PH, Procopic PG, et al. Childhood chronic inflammatory demyelinating polyneuropathy: Clinical course and long-term outcome. Neuromuscul Disord. 2000;10:398-406.
- Mahboubi S, Morris MC. Imaging of spinal infections in children. Radiol Clin North Am. 2001;39:215-222.
- Weinstein SL. The Pediatric Spine: Principles and Practice. New York: Raven Press; 1994.
- Onofrio BM. Intervertebral discitis: Incidence, diagnosis, and management. Clin Neurosurg. 1980;27:481-516.
- du Lac P, Panuel M, Devred P, et al. MRI of disc space infection in infants and children. Report of 12 cases. Pediatr Radiol. 1990;20:175-178.
- Brown R, Hussain M, McHugh K, et al. Discitis in young children. J Bone Joint Surg Br. 2001;83:06-111.
- Andronikou S, Jadwat S, Douis H. Patterns of disease on MRI in 53 children with tuberculous spondylitis and the role of gadolinium. Pediatr Radiol. 2002;32:798-805.
- Numaguchi Y, Rigamonti D, Rothman M, et al. Spinal epidural abscess: Evaluation with gadolinium-enhanced MR imaging. Radiographics. 1993;13:545-559; discussion 559-560.
Citation
Infections and inflammatory conditions of the pediatric spine and spinal cord. Appl Radiol.
February 6, 2014